Question: Please help me answering this step by step, thanks!!! :) You can use EES or any other software to solve this problem NUMERICAL SOLUTION (You
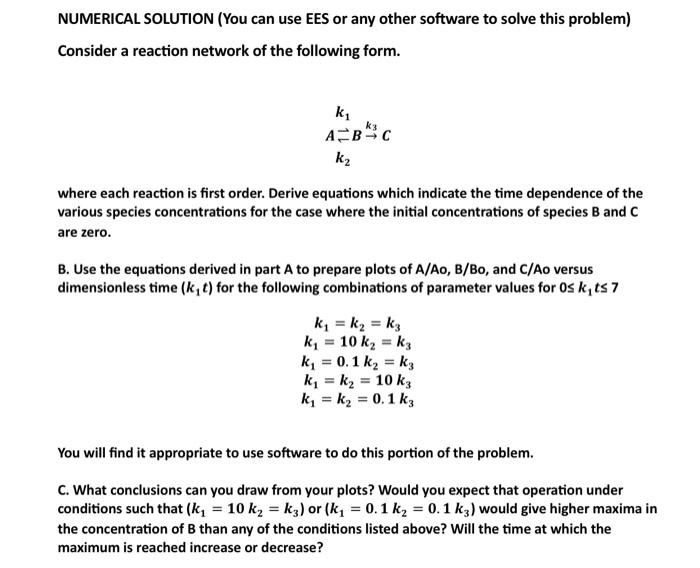
NUMERICAL SOLUTION (You can use EES or any other software to solve this problem) Consider a reaction network of the following form. k1ABk3Ck2 where each reaction is first order. Derive equations which indicate the time dependence of the various species concentrations for the case where the initial concentrations of species B and C are zero. B. Use the equations derived in part A to prepare plots of A/Ao,B/Bo, and C/Ao versus dimensionless time (k1t) for the following combinations of parameter values for 0k1t7 k1=k2=k3k1=10k2=k3k1=0.1k2=k3k1=k2=10k3k1=k2=0.1k3 You will find it appropriate to use software to do this portion of the problem. C. What conclusions can you draw from your plots? Would you expect that operation under conditions such that (k1=10k2=k3) or (k1=0.1k2=0.1k3) would give higher maxima in the concentration of B than any of the conditions listed above? Will the time at which the maximum is reached increase or decrease
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


