Question: please help me complete the table with shown work, the % labeled NaCIO and the w/v % * should* be 6% if lab was done


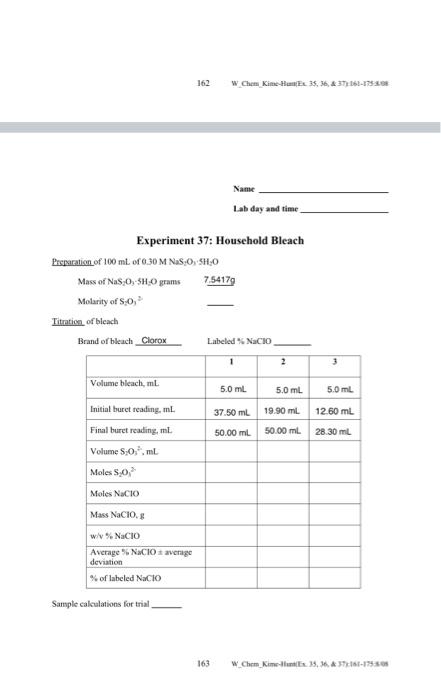
Experiment 37 The Oxidizing Power of a Household Bleach Household bleaches (Purer, Chlorox, etc.) usually contain the active ingredient sodium hypochlorite, an oxidizing agent to help remove stains. In this experiment we will determine the quantity of this oxidizing (hleaching agent in a sample of household bleach by reacting a known amount with excess potassium iodide, Kl, in acid solution. Hypochlorite hon oxidines the fodide ion to hodine according to the following redox reaction 21 + 1 + 2 2H + C + 2c + CrHO 2H+ + C10 + 2 = 1 + Cl + H2 The iodine liberated by this reaction is titrated with sodium thiosulfate, then the thiosalfate son reacts with the sodine in another redox reaction 1 +2621 25.0 S.O. + 2 25.0+1:21 + 0 From the quantity of sodium thiosulfate consumed, the weight percent of oxidizing power of the bleach can be calculated in terms of the grates of sodium hypochlorite per mL of bleach. The endpoint of an iodine titration is determined by adding such indicator which forms a nice, deep blue color with iodine that is easy to see. This color disappears when all of the iodine has reacted at the endpoint Procedure First, prepare 100 mL of 0.30 M NaS 0 (H:O) (FW - 248) in a suitable container, and use it in your buret. Then, place 50 ml of distilled water in a 250 ml. Erlenmeyer flask and add 2 gof KI, swirling the flask until the KI dissolves. Pipet 5.0 mL of bleach into the flask; swirl w Chen Kine Hex 35. 36.&37161-1988 161 until well-mixed and then add 20 ml. of 1 M HCl. A purple-brown color should appear, indicating the presence of sodine Titrate immediately with 0.30 sodium thisulfate solution until the brown color fades to yellow or light orange and then add 5 ml of starch indicator solution Slowly continue the titration until the deep blue-black color of the solution disappears Repeat the analysis with at least two more samples of the same bleach. For maximum accuracy. until well-mixed and then add 20 mL of 1 M HCL. A purple-brown color should appear indicating the peesence of iodine. Titrate immediately with 0.30 sodium thisultate solution until the brown color fades to yellow or light orange and then add 5 ml of starch indicator solution Slowly continue the titration until the deep blue-black color of the solution disappears. Repeat the analysis with at least two more samples of the same bleach For maximum accuracy. keep the bleach covered when not in use, or else the molarity of hypochlorite ion will decrease over time. The solution may be disposed in the sink with running water Calculations From the molarity and volume of added sodium thiosulfate, calculate the weight of the original bleach present in each s ml. sample, assuming it is NaCIO. Then, express each result as a (w/v/% of sodium hypochlorite in the beach. Report the average precision. Use the Q-test, if necessary. Compare your average with the value listed for the brand used 162 W Cem Kime Humax. 35. 36.4 373/61-1752.08 Name Lab day and time Fyneriment 37. Household Reach 162 w Chen Kim 35, 36, 37661-13:30 Name Lab day and time Experiment 37: Household Bleach Preparation of 100 mL of 0.30 M NSO, SH.0 Mass of Nas 0, 5H.0 grams 7.54179 Molarity of So, Titration of blcach Brand of bleach Clorox Labeled Nacio 1 2 3 Volume bleach, ml. 5.0 mL 5.0 ml. 5.0 mL 37.50 ml 19.90 mL 50.00 mL 50.00 mL 12.60 mL 28.30 mL Initial buret reading, ml Final buret reading, ml. Volume 80.,ml Moles so, Moles Nacio Mass NaCIO w/V% NaCIO Average NACIOs average deviation %of labeled Nacio Sample calculations for trial 163 W Chem_kim35, 36, 37161-19:50
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


