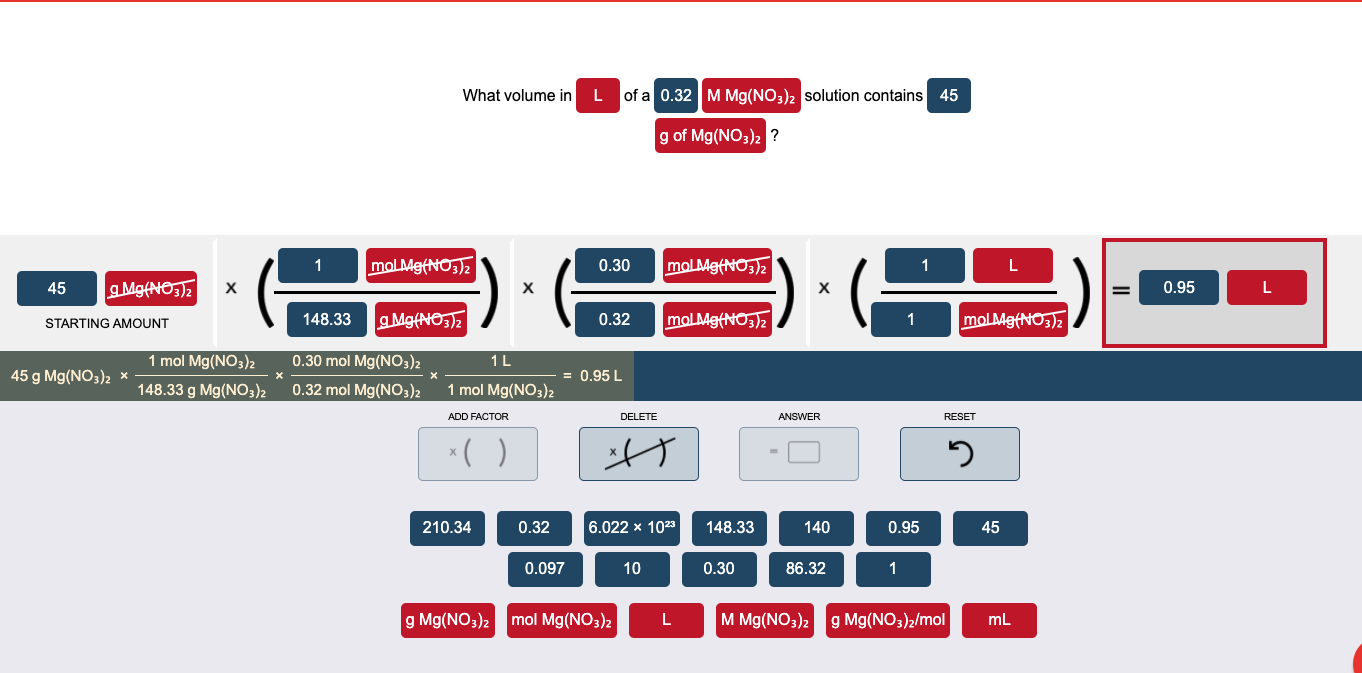
Question: please help me do the dimensional analysis to get the right answer. What volume in L of a 0.32 M Mg(NO3)2 solution contains 45 g
 please help me do the dimensional analysis to get the right answer.
please help me do the dimensional analysis to get the right answer.
What volume in L of a 0.32 M Mg(NO3)2 solution contains 45 g of Mg(NO3)2 ? 1 mol Mg(NO3)2 0.30 mol Mg(NO3)2 1 L 45 g Mg(NO3)2 0.95 L STARTING AMOUNT 148.33 g Mg(NO3)2 0.32 mol Mg(NO3)2 1 mol Mg(NO3)2 1L 45 g Mg(NO3)2 * mol Mg(NO3)2 148.33 g Mg(NO3)2 0.30 mol Mg(NO3)2 0.32 mol Mg(NO3)2 = 0.95 L 1 mol Mg(NO3)2 ADD FACTOR DELETE ANSWER RESET * ( ) xH 2 210.34 0.32 6.022 x 1023 148.33 140 0.95 45 0.097 10 0.30 86.32 1 g Mg(NO3)2 mol Mg(NO3)2 L M Mg(NO3)2 g Mg(NO3)2/mol mL
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


