Question: Please help me how to find the QC+200ppm ( using Standard Addition Method) and its Error which is highlighted at the bottom of the table?
Please help me how to find the QC+200ppm ( using Standard Addition Method) and its Error which is highlighted at the bottom of the table?
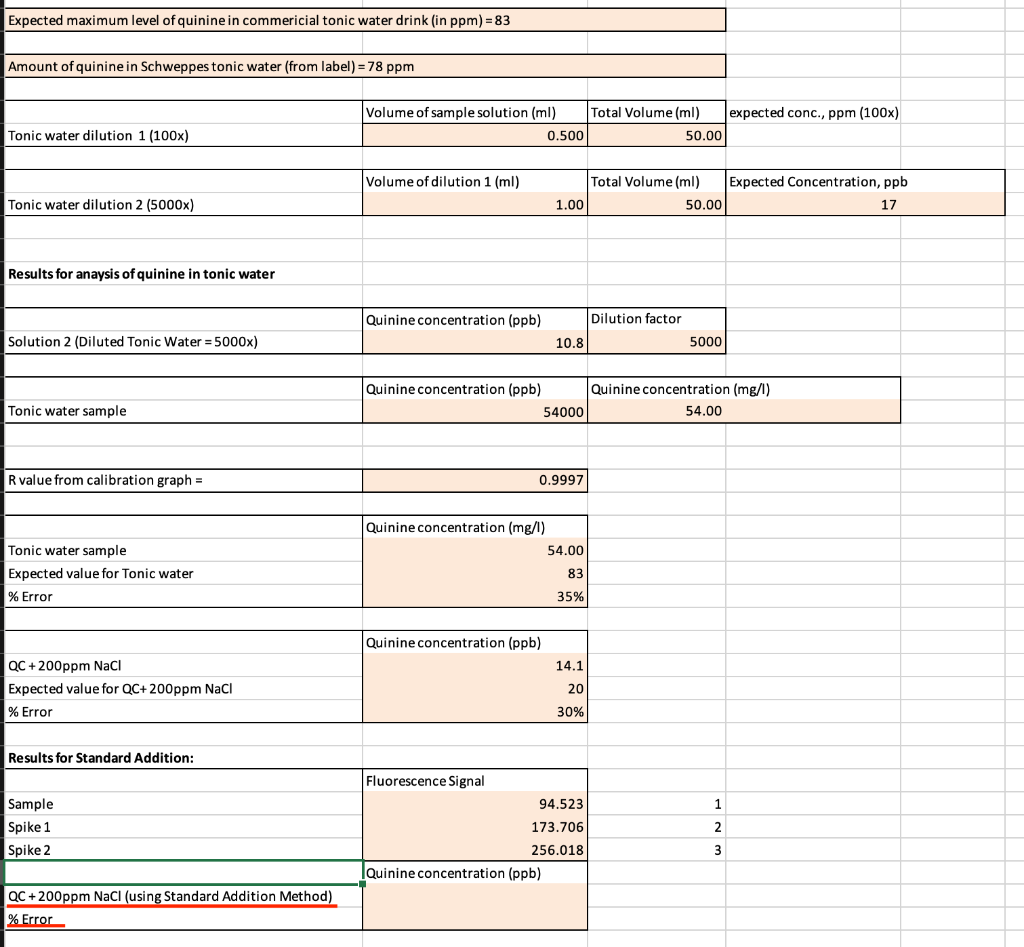
Expected maximum level of quinine in commericial tonic water drink (in ppm)=83 Amount of quinine in Schweppes tonic water (from label) = 78 ppm Volume of sample solution (ml) Total Volume (ml) expected conc., ppm (100x) 0.500 50.00 Tonic water dilution 1 (100x) Volume of dilution 1 (ml) Total Volume (ml) Expected Concentration, ppb 1.00 50.00 17 Tonic water dilution 2 (5000x) Results for anaysis of quinine in tonic water Quinine concentration (ppb) Dilution factor Solution 2 (Diluted Tonic Water = 5000x) 10.8 5000 Quinine concentration (ppb) 54000 Quinine concentration (mg/l) 54.00 Tonic water sample R value from calibration graph = 0.9997 Quinine concentration (mg/l) 54.00 Tonic water sample Expected value for Tonic water % Error 83 35% Quinine concentration (ppb) 14.1 QC + 200ppm Naci Expected value for QC+200ppm Naci % Error 20 30% Results for Standard Addition: 1 Sample Spike 1 Spike 2 Fluorescence Signal 94.523 173.706 256.018 Quinine concentration (ppb) 2 wN QC +200ppm NaCl (using Standard Addition Method) % Error Expected maximum level of quinine in commericial tonic water drink (in ppm)=83 Amount of quinine in Schweppes tonic water (from label) = 78 ppm Volume of sample solution (ml) Total Volume (ml) expected conc., ppm (100x) 0.500 50.00 Tonic water dilution 1 (100x) Volume of dilution 1 (ml) Total Volume (ml) Expected Concentration, ppb 1.00 50.00 17 Tonic water dilution 2 (5000x) Results for anaysis of quinine in tonic water Quinine concentration (ppb) Dilution factor Solution 2 (Diluted Tonic Water = 5000x) 10.8 5000 Quinine concentration (ppb) 54000 Quinine concentration (mg/l) 54.00 Tonic water sample R value from calibration graph = 0.9997 Quinine concentration (mg/l) 54.00 Tonic water sample Expected value for Tonic water % Error 83 35% Quinine concentration (ppb) 14.1 QC + 200ppm Naci Expected value for QC+200ppm Naci % Error 20 30% Results for Standard Addition: 1 Sample Spike 1 Spike 2 Fluorescence Signal 94.523 173.706 256.018 Quinine concentration (ppb) 2 wN QC +200ppm NaCl (using Standard Addition Method) % Error
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


