Question: Please help me label and summarize the results and analysis of the IR/NMR. IR NMR Nitration of Methyl Benzoate HNO3/H2SO4 Methyl benzoate Methyl 3-nitrobenzoate Introduction
Please help me
label and summarize the results and analysis of the IR/NMR.
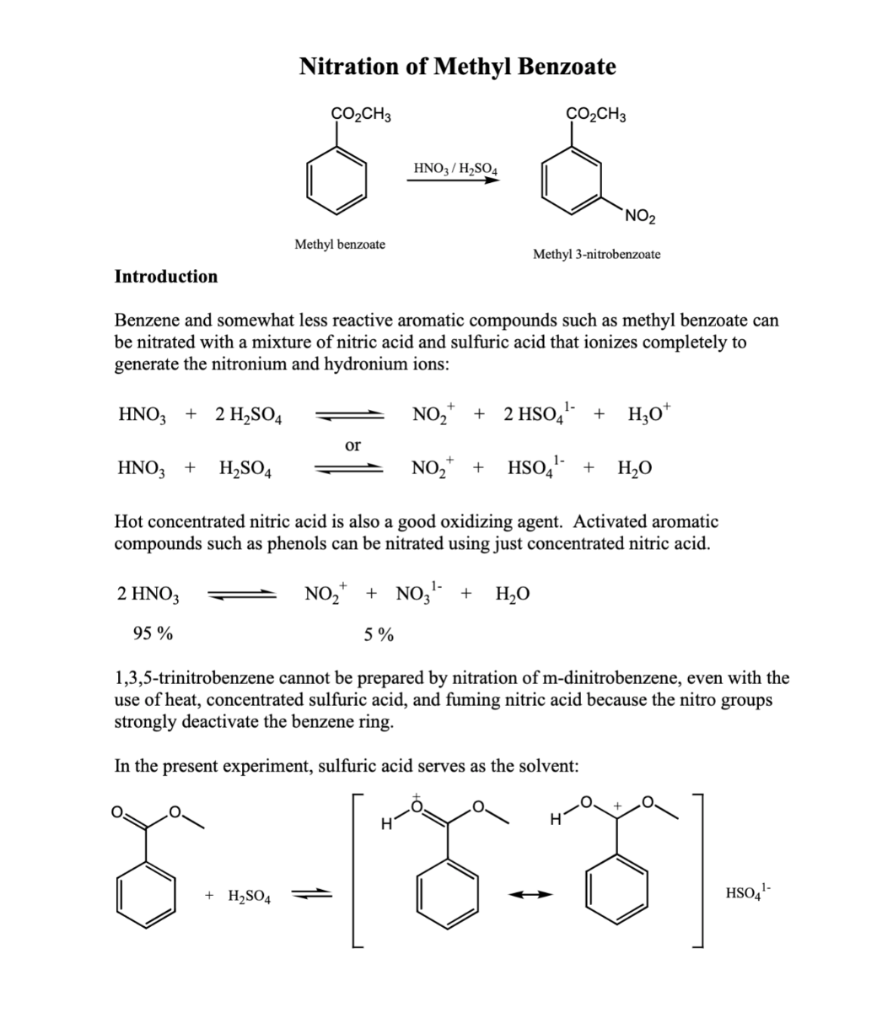
IR
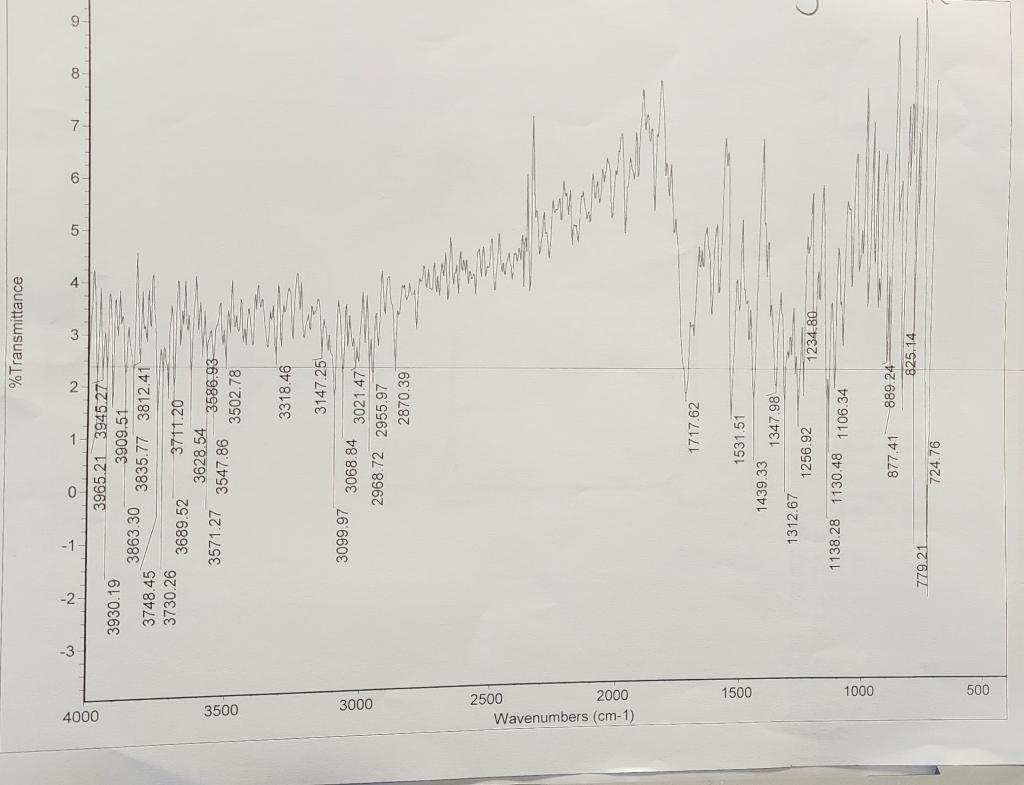
NMR
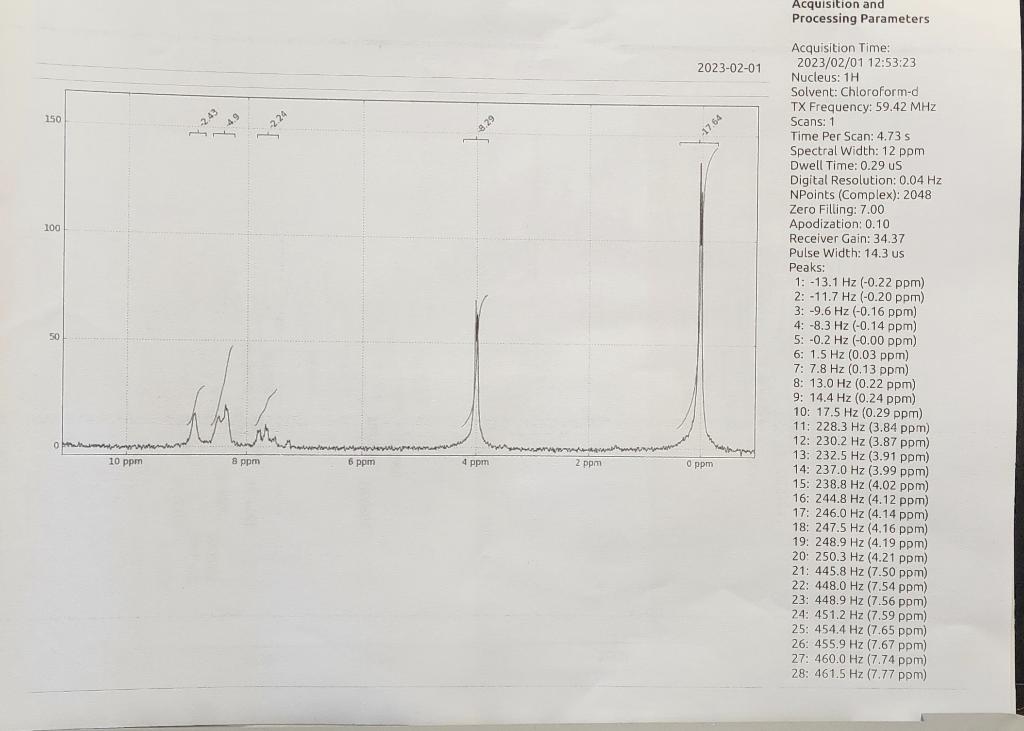
Nitration of Methyl Benzoate HNO3/H2SO4 Methyl benzoate Methyl 3-nitrobenzoate Introduction Benzene and somewhat less reactive aromatic compounds such as methyl benzoate can be nitrated with a mixture of nitric acid and sulfuric acid that ionizes completely to generate the nitronium and hydronium ions: HNO3+2H2SO4NO2++2HSO41+H3O+HNO3+H2SO4+NO2++HSO41+H2O Hot concentrated nitric acid is also a good oxidizing agent. Activated aromatic compounds such as phenols can be nitrated using just concentrated nitric acid. 2HNO3NO2++NO31+H2O95%5% 1,3,5-trinitrobenzene cannot be prepared by nitration of m-dinitrobenzene, even with the use of heat, concentrated sulfuric acid, and fuming nitric acid because the nitro groups strongly deactivate the benzene ring. In the present experiment, sulfuric acid serves as the solvent: +H2SO4 HSO41 Acquisition and Processing Parameters Acquisition Time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


