Question: please help me out with the ones i got incorrect! and my eauilibrium constant sas 0 for the last question. initial concentration of acetic acid


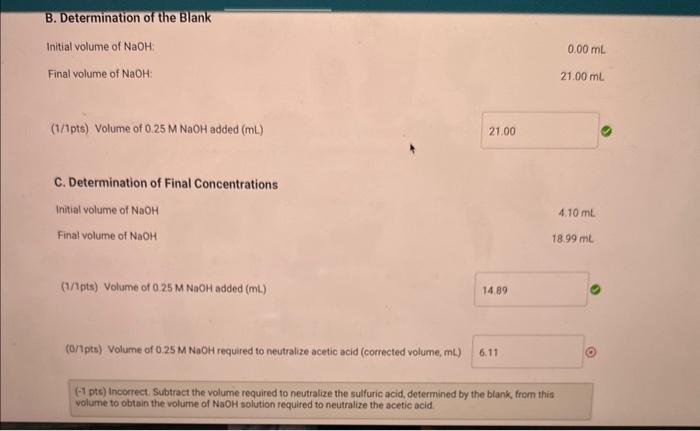
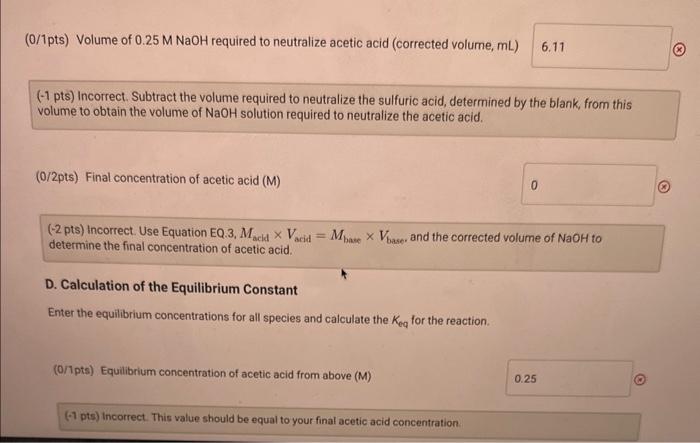

A. Determination of initial concentrations Part A: Initial buret reading (mL) Part A: Final buret reading (mL) B. Determination of the Blank Part B: Initial buret reading ( mL) Part B: Final buret reading ( mL ) 0.0021.00 C. Determination of Final Concentrations Part C: Initial buret reading (mL) Part C: Final buret reading ( mL) A. Determination of Initial Concentrations Initial volume of NaOH : Final volume of NaOH : (1/1pts) volume of 0.25MNaOH added ( mL ) (0/2pts) initiol concentration of acetic acid (M) (-2 pts) Incorrect. Use Equation EQ.3, MwidVsedd=MbusVbeme. to determine the initial concentration of acetic acid. B. Determination of the Blank Initial volume of NaOH Final volume of NaOH : 21.00mL (1/1pts) Volume of 0.25MNaOH added (mL) 21.00 C. Determination of Final Concentrations Initial volume of NaOH Final volume of NaOH 4.10mL 18.99mL (1/1pts) Volume of 0.25MNaOH added (mL) 14.89 (0/1pts) Volume of 0.25MNoOH required to neutralize acetic acid (corrected volume, mL)6.11 (-1 pts) incorrect, Subtract the volume required to neutralize the sulfuric acid, determined by the blank from this volume to obtoin the volume of NaOH solution required to neutralize the acetic acid. (0/1pts) Volume of 0.25MNaOH required to neutralize acetic acid (corrected volume, mL ) (1 pts) Incorrect. Subtract the volume required to neutralize the sulfuric acid, determined by the blank, from this volume to obtain the volume of NaOH solution required to neutralize the acetic acid. (0/2pts) Final concentration of acetic acid (M) (-2 pts) incorrect. Use Equation EQ.3, MaclaVacid=MbaseVbase, and the corrected volume of NaOH to determine the final concentration of acetic acid. D. Calculation of the Equilibrium Constant Enter the equilibrium concentrations for all species and calculate the Keq for the reaction. (0/1pts) Equilibrium concentration of acetic acid from above (M) 1. Examine the magnitude of your calculated equilibrium constant, Keq. Does the equilibrium favor reactants or products? Explain your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


