Question: Please help me out with this pre lab assignment. D. Relative Scales of Reduction and Oxidation Potentials ranking of ions by ease of reduction, easiest

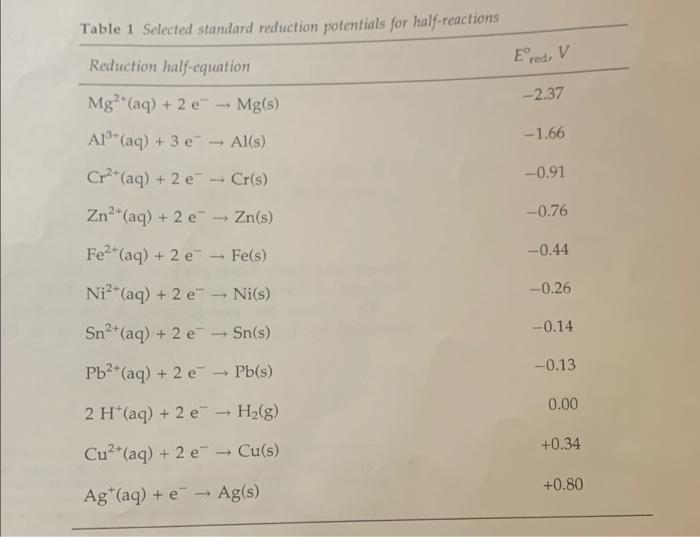
D. Relative Scales of Reduction and Oxidation Potentials ranking of ions by ease of reduction, easiest to hardest: * comparison of ranking with ranking based on reduction potentials in Table 1: ranking of ions by ease of oxidation, easiest to hardest: * comparison of ranking with ranking based on oxidation potentials in Table 1: Table 1 Selected standard reduction potentials for half-reactions Ered, V -2.37 Reduction half-equation Mg?"(aq) + 2 e" - Mg(s) Al(aq) + 3 e Al(s) Cr2+ (aq) + 2e - Cr(s) -1.66 -0.91 -0.76 -0.44 -0.26 Zn2+ (aq) + 2 e - Zn(s) Fe2+ (aq) + 2 e - Fe(s) Ni2+ (aq) + 2 e-Ni(s) Sn(aq) + 2 e - Sn(s) Pb2+ (aq) + 2 e Pb(s) -0.14 -0.13 - 0.00 - 2 H(aq) + 2 H2(g) *e Cu2+ (aq) + 2 e - Cu(s) +0.34 +0.80 Ag+(aq) + e - Ag(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


