Question: Please help me solve for these two problems. If you answer them correctly, I will give you a thumbs up(: For A - products, time
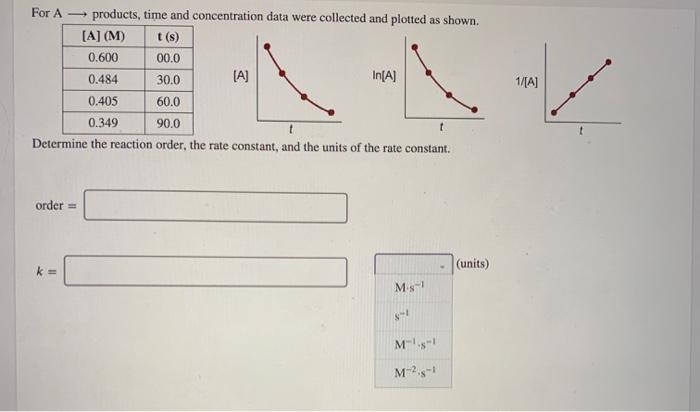

For A - products, time and concentration data were collected and plotted as shown. [A] (M) t(s) 0.600 00.0 0.484 30.0 [A] In[A] 0.405 60.0 0.349 90.0 Determine the reaction order, the rate constant, and the units of the rate constant. 1/[A] t order = (units) k= M. M-5-1 M2.5-1 A certain reaction has an activation energy of 54.87 kJ/mol. At what Kelvin temperature will the reaction proceed 8.00 times faster than it did at 353 K? T= K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


