Question: Please help me solve these. Thank you. The equilibrium constant, Kc, for the following reaction is 1.66102 at 668K. 2HI(g)?H2(g)+I2(g) Calculate Kc at this temperature
Please help me solve these. Thank you.

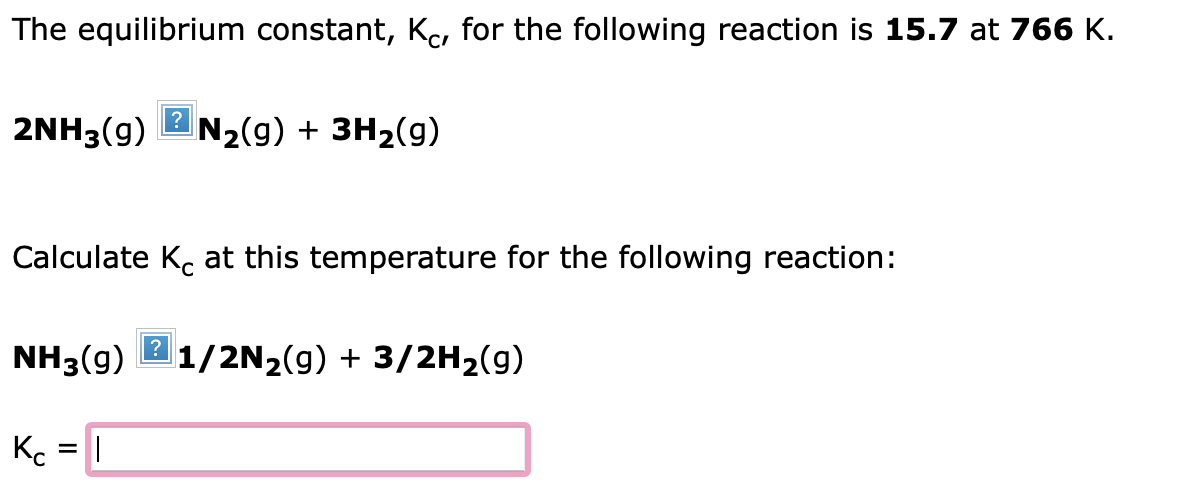
The equilibrium constant, Kc, for the following reaction is 1.66102 at 668K. 2HI(g)?H2(g)+I2(g) Calculate Kc at this temperature for: H2(g)+I2(g)2HI(g) Kc= The equilibrium constant, Kc, for the following reaction is 15.7 at 766K. 2NH3(g)N2(g)+3H2(g) Calculate Kc at this temperature for the following reaction: NH3(g)1/2N2(g)+3/2H2(g) Kc=1
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


