Question: please help me solve this. Table 1: Hydrogen gas emission spectrum Wavelength Intensity Violet spectral line 410 nm faint Choose... moderate 420 Blue-violet spectral line
please help me solve this.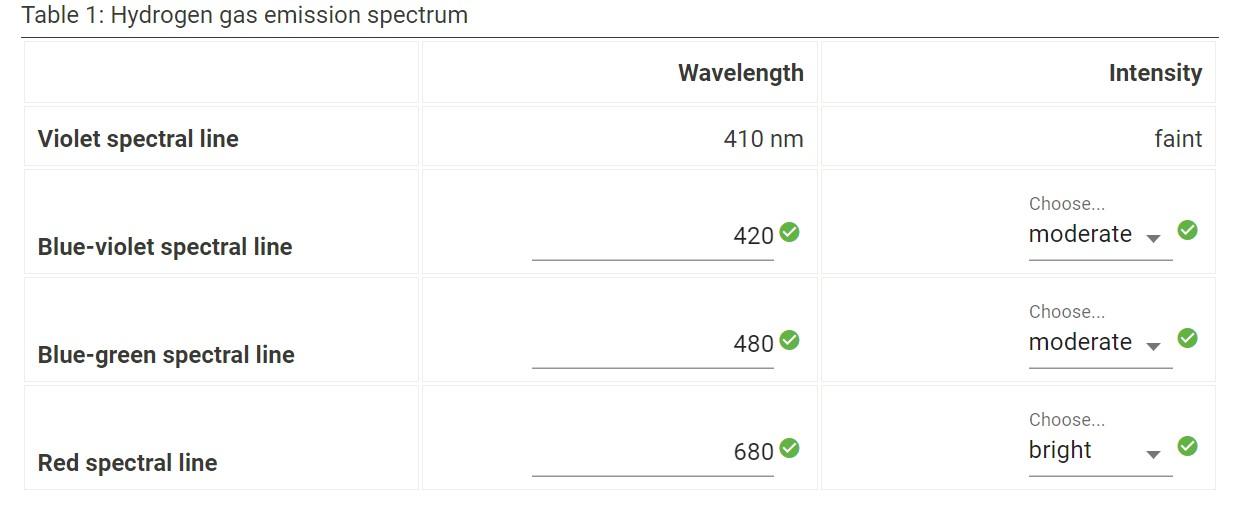

Table 1: Hydrogen gas emission spectrum Wavelength Intensity Violet spectral line 410 nm faint Choose... moderate 420 Blue-violet spectral line Choose... moderate 480 Blue-green spectral line Choose... bright 680 Red spectral line Calculate the n for the three strongest of the lines in the hydrogen spectrum (2.5pts) The magnitude of AEatom for the blue-violet wavelength identified in part 2 (2.5pts) n for the blue-violet wavelength (2.5pts) The magnitude of Eatom for the blue-green wavelength identified in part 2 (2.5pts) ni for the blue-green wavelength (2.5pts) The magnitude of Eatom for the red wavelength identified in part 2 (2.5pts) n, for the red wavelength
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


