Question: Please help me to solve this problem correctly and detailed. Thank you. 4. Consider the chemical vapor deposition (CVD) process for the manufacture of solid
Please help me to solve this problem correctly and detailed. Thank you.
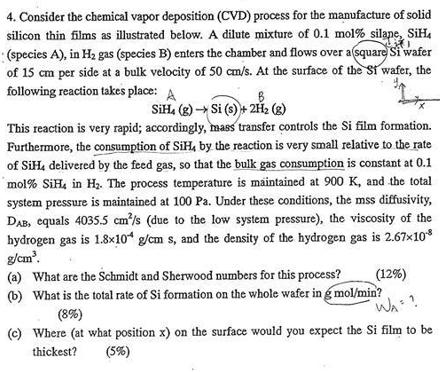
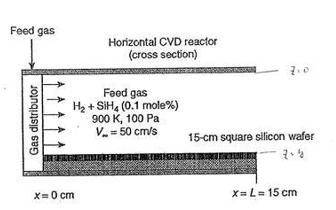
4. Consider the chemical vapor deposition (CVD) process for the manufacture of solid silicon thin films as illustrated below. A dilute mixture of 0.1 mol% silape, SiH (spccies A), in H2 gas (species B) enters the chamber and flows over a square Si wafer of 15 cm per side at a bulk velocity of 50 cm/s. At the surface of the st wafer, the following reaction takes place: A B SiH. (g) Si (3) + 2H2 () This reaction is very rapid; accordingly, mass transfer controls the Si film formation. Furthermore, the consumption of SiH, by the reaction is very small relative to the rate of SiHdelivered by the feed gas, so that the bulk gas consumption is constant at 0.1 mol% SiH, in Hz. The process temperature is maintained at 900 K, and the total system pressure is maintained at 100 Pa. Under these conditions, the mss diffusivity, DAB, equals 4035.5 cm/s (due to the low system pressure), the viscosity of the hydrogen gas is 1.8*10* g/cm s, and the density of the hydrogen gas is 2.67x108 g/cm (a) What are the Schmidt and Sherwood numbers for this process? (12%) 6) What is the total rate of Si formation on the whole wafer in g mol/min? (8%) (1) Where (at what position x) on the surface would you expect the Si film to be thickest? (5%) WA? Feed gas Horizontal CVD reactor (cross section) 7. Gas distributor Feed gas Hz + SiH, (0.1 mole%) 900 K, 100 Pa V50 cm/s 15-cm square silicon wafer X=0 cm X=L= 15 cm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


