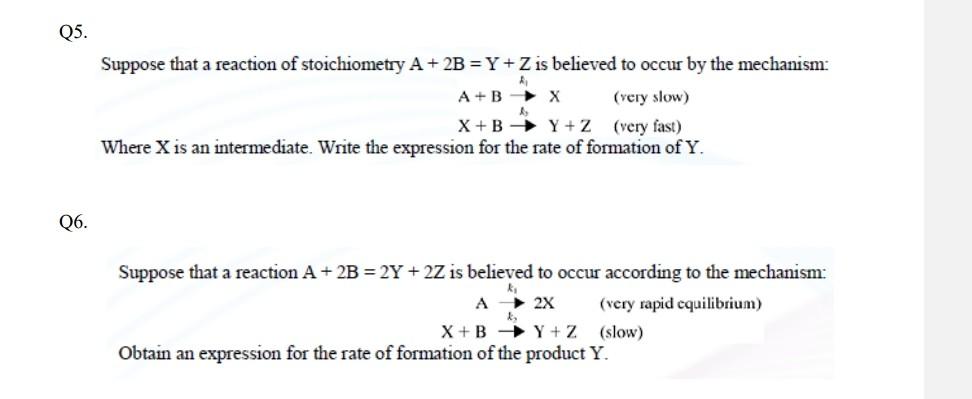
Question: Please help me to solve this problems as fast as possible, thanks a lot 25. Suppose that a reaction of stoichiometry A+2B=Y+Z is believed to
Please help me to solve this problems as fast as possible, thanks a lot
25. Suppose that a reaction of stoichiometry A+2B=Y+Z is believed to occur by the mechanism: A+Bk^1X(veryslow)X+BlY+Z(veryfast) Where X is an intermediate. Write the expression for the rate of formation of Y. 26. Suppose that a reaction A+2B=2Y+2Z is believed to occur according to the mechanism: Obtain an expression for the rate of formation of the product Y
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


