Question: please help me. what is the formula I use for these and what numbers get plugged in where? + First Order Plot -101 - -11
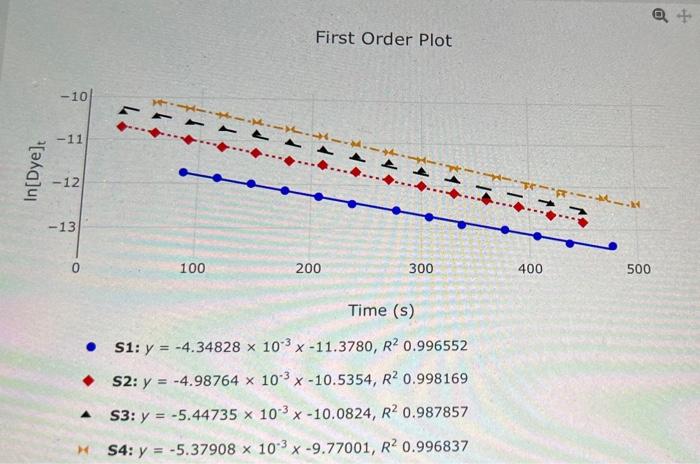
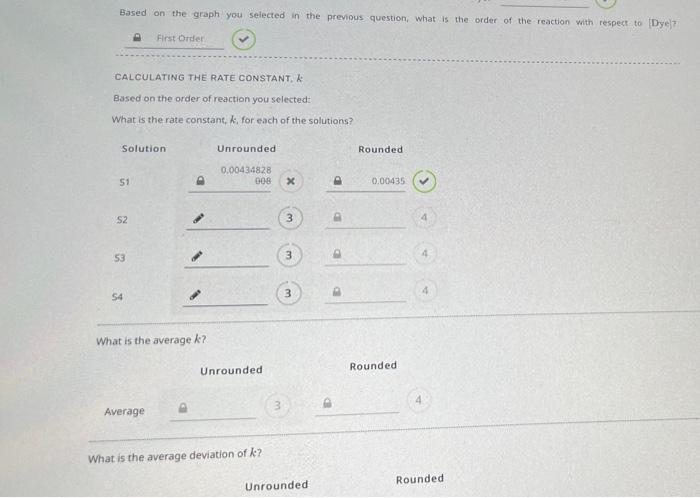
+ First Order Plot -101 - -11 In[Dye) -12 .. -13 100 200 300 400 500 Time (s) S1: y = -4.34828 x 103 x -11.3780, R2 0.996552 S2: y = = -4.98764 x 103 x -10.5354, R2 0.998169 A S3: y = -5.44735 x 10 x -10.0824, R? 0.987857 S4: y = -5.37908 x 10 x 19.77001, R? 0.996837 Based on the graph you selected in the previous question, what is the order of the reaction with respect to Dye? First Order CALCULATING THE RATE CONSTANT Based on the order of reaction you selected: What is the rate constant, k, for each of the solutions? Solution Unrounded Rounded 0.00434828 008 51 X 0.00435 S2 3 53 3 . 4 3 54 3 What is the average k? Unrounded Rounded 3 Average What is the average deviation of ke? Rounded Unrounded
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


