Question: Please help me with ANSWER B. Also let me know if A is right. 6. As discussed in class, Oxygen (atomic # =8 ) has
Please help me with ANSWER B. Also let me know if "A" is right.
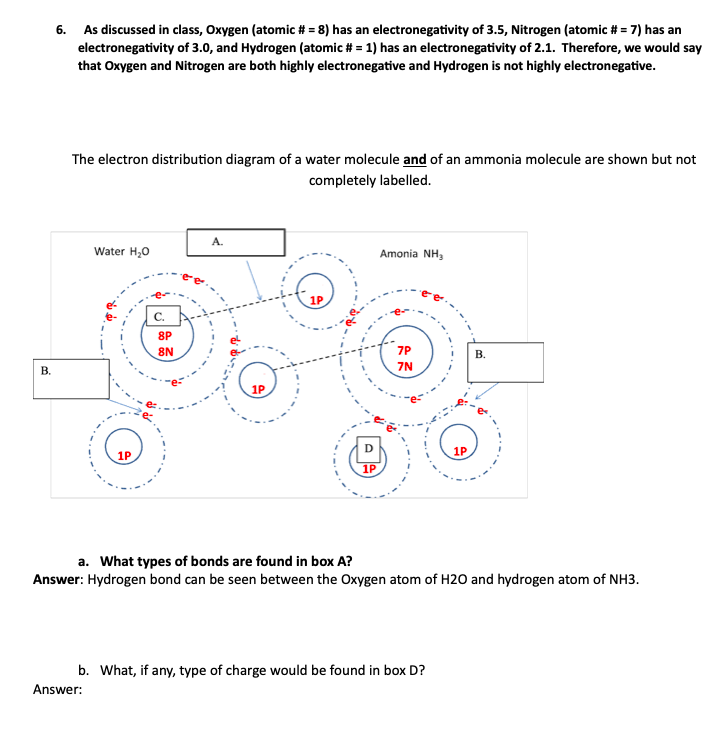
6. As discussed in class, Oxygen (atomic \# =8 ) has an electronegativity of 3.5, Nitrogen (atomic \# =7 ) has an electronegativity of 3.0, and Hydrogen (atomic \# = 1) has an electronegativity of 2.1. Therefore, we would say that Oxygen and Nitrogen are both highly electronegative and Hydrogen is not highly electronegative. The electron distribution diagram of a water molecule and of an ammonia molecule are shown but not completely labelled. a. What types of bonds are found in box A? Answer: Hydrogen bond can be seen between the Oxygen atom of H2O and hydrogen atom of NH3. b. What, if any, type of charge would be found in box D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


