Question: please help me with that prelab Table 5.3. Rx values of the spice extracts in different mobile phases Roof Cinnam- aldehyde Rrof Anethole Roof Vanillin
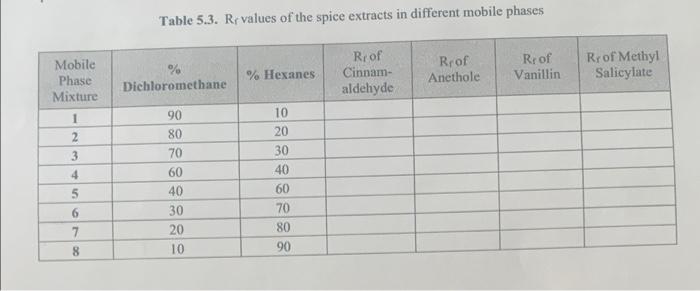



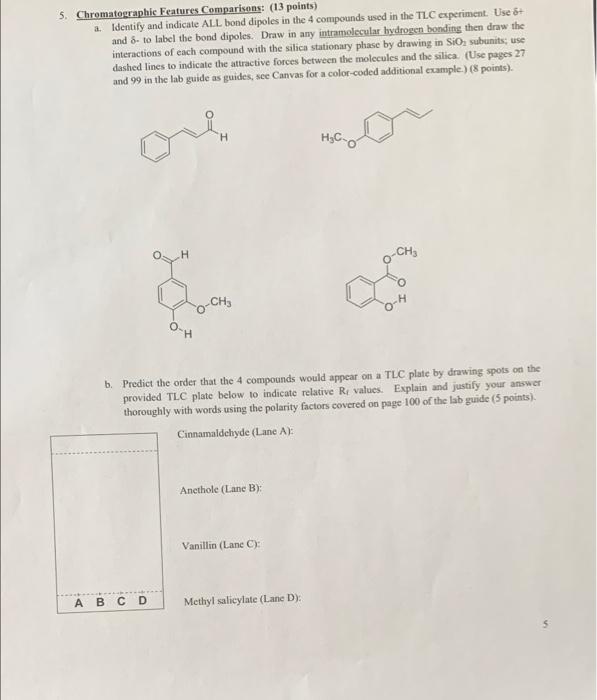
Table 5.3. Rx values of the spice extracts in different mobile phases Roof Cinnam- aldehyde Rrof Anethole Roof Vanillin % Hexanes Rr of Methyl Salicylate Dichloromethane Mobile Phase Mixture 1 2 3 4 90 80 70 60 40 30 20 10 10 20 30 40 60 70 80 90 5 6 7 8 3. Prelab Exercise Questions: (21 points) a. Why do non-polar mobile phases (like hexanes) result in lower Rr values of analytes on a silica TLC plate? Why do polar mobile phases (like dichloromethane) increase Ry values of analytes on a silica TLC plate? Explain using justifications with adsorbed vs, free states. (4 points) b. A student spots a standard in Lane 1, an unknown in Lane 3, and a co-spot in Lane 2 on a TLC plate and develops it using 100% ethyl acetate as the mobile phase. Only one spot was observed in all three lanes with an R value - 0.99 as seen to the left (using UV light), i. Does this TLC plate indicate that the unknown material matches with the standard (yes or no)? (1 point) ii. Does this TLC plate indicate that the unknown is only a single compound (yes or no)? (1 point) iii. Explain your answers! If yes - justify why. If no - what can be done to improve the results of this TLC plate? (2 points) ------ c. What's wrong with the TLC plate shown on the lef? What caused this issue? How can it be fixed? Lane 1 - standard: Lane 2 - co-spot; Lane 3 - an unknown compound. (4 points) 110 ------ 2 d. Why is a co-spot lane so important to use when running TLC? Explain thoroughly. (3 points) e. Use the four molecules below (A through D) to answer the next two questions: i. Put the following molecules in order from least polar to most polar. (2 points) OH OH Dodo com o B D least polar most polar ii. Below is a TLC plate with four lanes. One compound (A, B, C, or D) has been spotted in each lane. Match the lanes to each compound by completing the table below. Calculate the R value for each compound, write your answer in the proper column in the table. Use a ruler to do your measurements. Be sure to show ALL R, value calculations! (4 points) 1 2 3 4 Compound Calculated Lane Identity RiValue (A, B, C, or D) Lane 1 Lane 2 Lane 3 Lane 4 --------- 3 4. REACTION TLC ANALYSIS (12 points): The reaction of maleic anhydride and aniline depicted below is an example of a nucleophilic acyl substitution. The product, an amide, is formed in three clementary steps: nucleophilic addition, nucleophile elimination, and a proton transfer. Using the synthesis depicted below, answer the following questions: . diethyl ether NH . a. maleic anhydride aniline maleanlinic acid mp 53C bp 184C mp 193C Predict the order of compounds 1, 2, and 3 on a TLC plate and draw spots in the labeled lancs on the TLC plate below to indicate relative R values (the mobile phase is 25% ethyl acetate/75% hexane, all can be visualized by UV lamp and/or an iodine chamber). Explain and justify your answer thoroughly with words using the polarity factors covered on page 100 of the lab guide. An example of how we want you to answer this question can be found on page 29 of the lab guide (9 points) Compound 1 (Lane 1) Compound 2 (Lane 2) 1 2 3 Compound 3 (Lane 3) b. What would happen to the location and/or order of the spots on the TLC plate if you changed the mobile phase to 75% ethyl acetate/25% hexane? Draw the spots on the NEW TLC plate (right) to indicate relative Ry values and justify your answer in words); be sure to discuss free vs. adsorbed state of the molecules on the silica. (3 points) 1 2 3 5. Chromatographie Features Comparisons: (13 points) a. Identify and indicate ALL bond dipoles in the 4 compounds used in the TLC experiment. Use 8+ and 8- to label the bond dipoles. Draw in any intramolecular hydrogen bonding then draw the interactions of each compound with the silica stationary phase by drawing in SiO, subunits, use dashed lines to indicate the attractive forces between the molecules and the silica (Use pages 27 and 99 in the lab guide as guides, see Canvas for a color-coded additional example.) (8 points) HC CH CHE b. Predict the order that the 4 compounds would appear on a TLC plate by drawing spots on the provided TLC plate below to indicate relative Re values. Explain and justify your answer thoroughly with words using the polarity factors covered on page 100 of the lab guide (5 points). Cinnamaldehyde (Lane A) Ancthole (Lane B): Vanillin (Lane C): A B C D Methyl salicylate (Lane D)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


