Question: please help me with these. Thank you!! Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed,
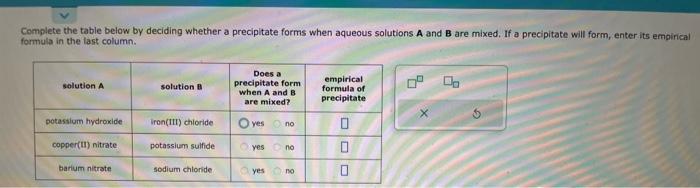
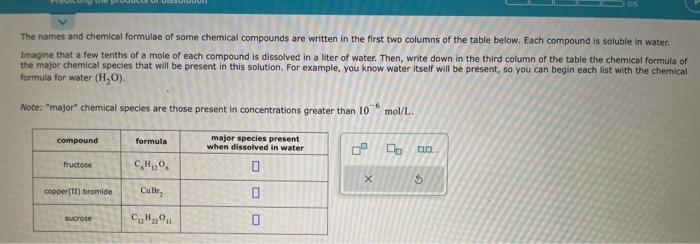
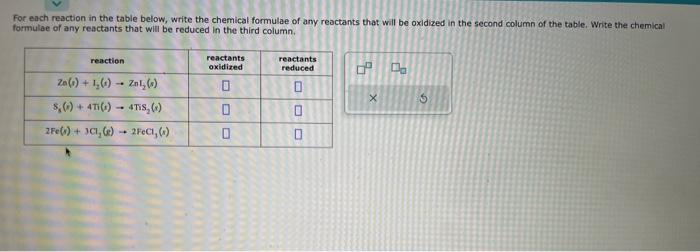
Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed, If a precipitate will form, enter its empinical formula in the tast column. The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound is soluble in water. Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. Then, write down in the third column of the table the chemical formula of the major chemical species that will be present in this solution. For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H2O). Note: "major" chemical species are those present in concentrations greater than 106mol/L.. For each reaction in the table below, write the chemical formulae of any reactants that will be oxidized in the second column of the table. Write the chemical formulae of any reactants that will be reduced in the third column
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


