Question: Please help me with this assignment. I've attached the document and the site to answer these questions is: https://phet.colorado.edu/en/simulations/under-pressure PHY213 Physics I PHY213 Online Lab
Please help me with this assignment. I've attached the document and the site to answer these questions is: https://phet.colorado.edu/en/simulations/under-pressure



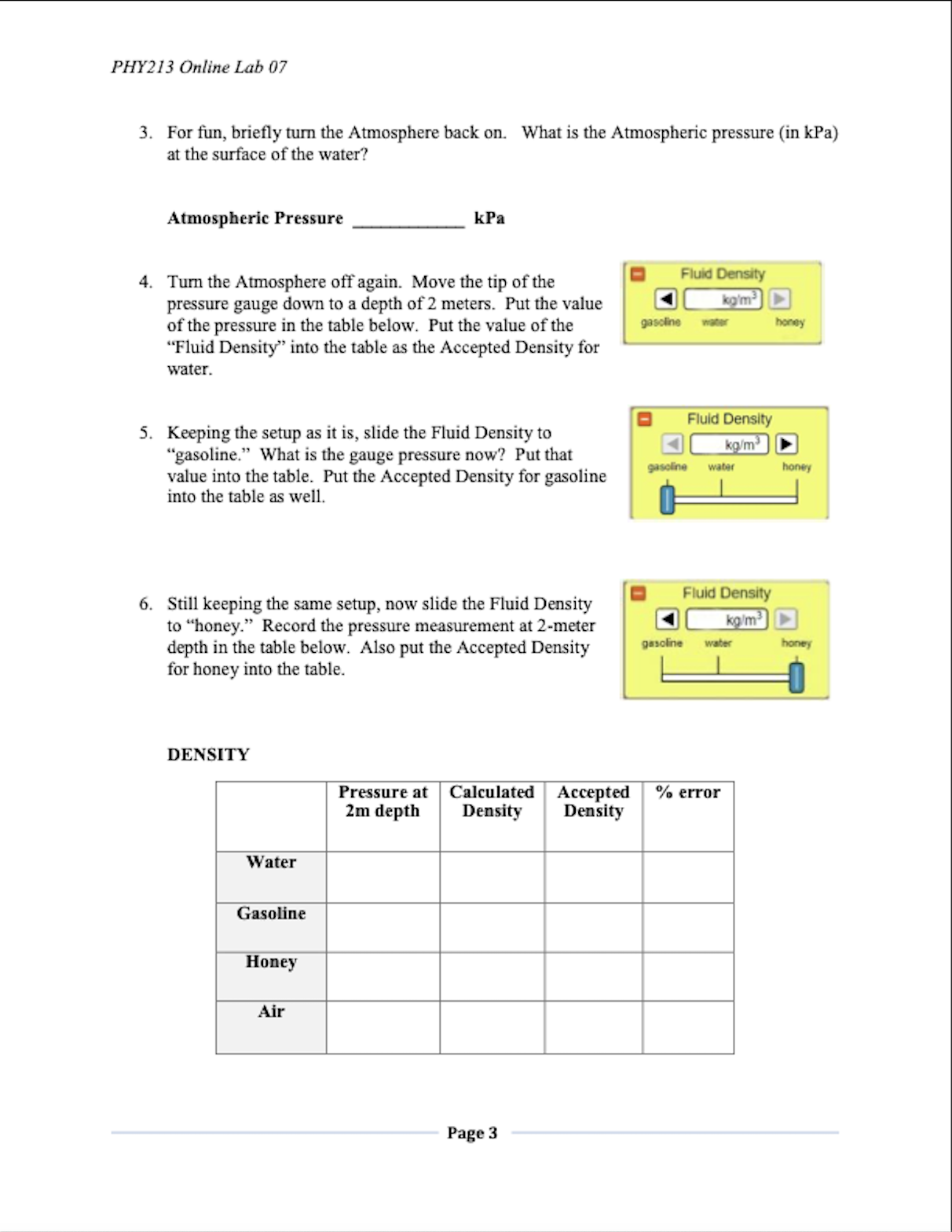

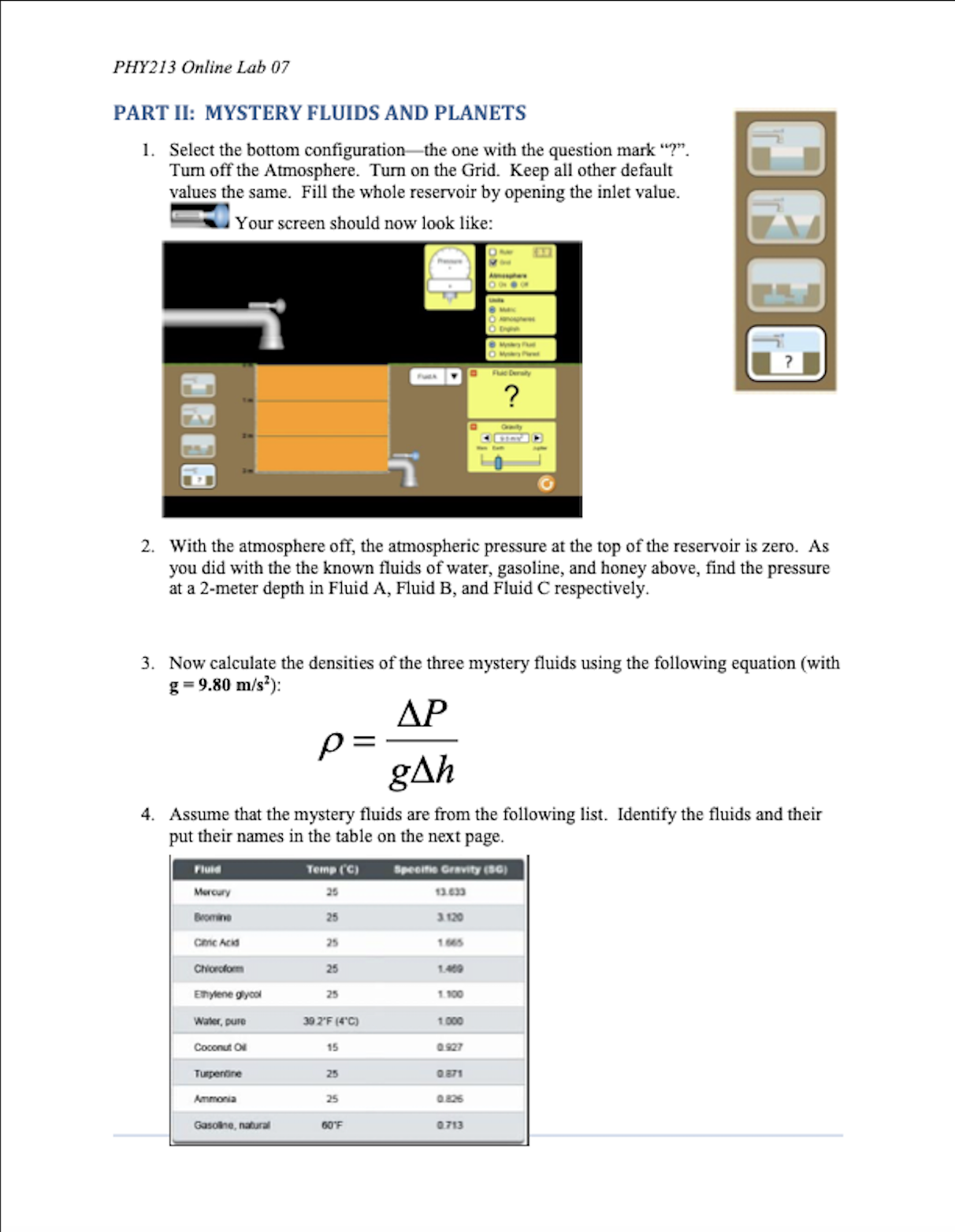

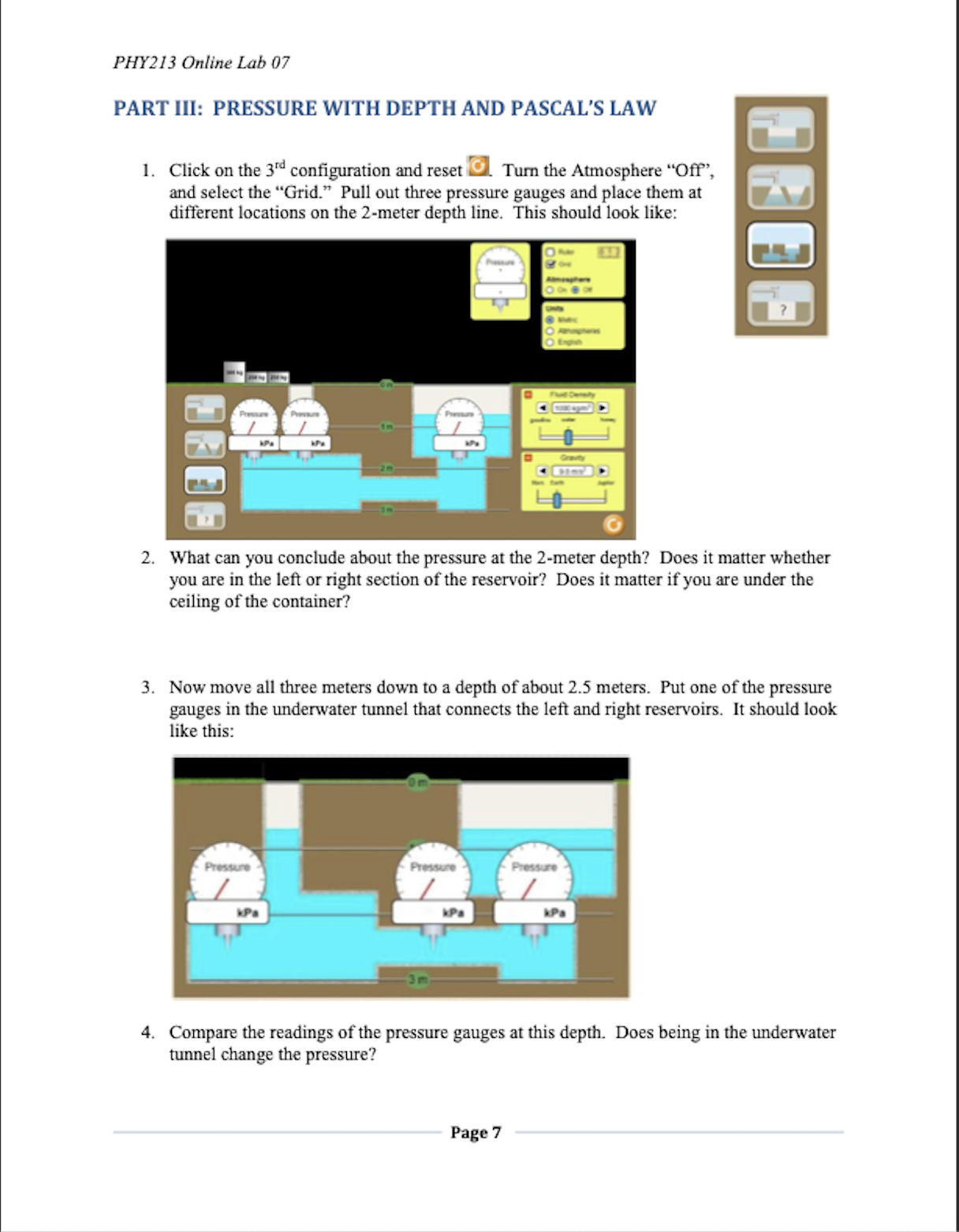


PHY213 Physics I PHY213 Online Lab 07 Lab 07: Under Pressure PURPOSE In this experiment we will investigate Pressure. You will use an online simulation from the University of Colorado, Boulder, called Under Pressure THEORY The Pressure (Force per Area) within a fluid is described by the equation: P = P. + pgh Where P. is the pressure at the surface (usually Atmospheric pressure), p is the density of the fluid, h is the depth within the fluid, and g is the acceleration due to gravity. The "Gauge pressure" is the pressure relative to atmospheric pressure. In a static fluid where the absolute pressure will only increase with depth, the gauge pressure is positive and is given by: P. = pgh Using this, if we measure two points within a static fluid, we find that the density of the fluid is: AP p= gAh Where AP is the pressure difference between the two points, and Ah is the depth difference. Finally, Pascal's Law states that a pressure (Force per Area) applied to an enclosed fluid is felt all the way through that fluid: AP = APB OBJECTIVES In this experiment, you will . 1. Calculate density for several fluids. . 2. Use fluids to calculate the gravity of some "Mystery" planets. . 3. Investigate Pascal's Law. Page 1PHY213 Online Lab 07 CONCLUSION Write a conclusion summarizing the results of this experiment. Be sure to 1. Restate the purpose, 2. State the main data values that pertain to the purpose, 3. State the % error on these main data values (if applicable), and 4. State at least three sources of error in the experiment. When you have filled in all of your data, answered the questions, and thought about the conclusion, you are ready to save to take Lab Quiz 07. Page 10PHI?! 3 Oniine Lab 07 SETUP load the "Under Pressure" simulation by either clicking on the above link1 or by clicking on the link in the lab folder in Blackboard. Your initial screen should look like the gure below: PART I: FLUID DENSITY I. Keep "Units" on Metric. Turn off the Atmosphere. Turn on the Grid. Keep all other default values the same. Fill the whole reservoir by opening the inlet value. B Your screen should now look like: 2. We could have left the Atmosphere on, and then subtracted that prasure nm all of our measurements. But turning it off will make the measurements a little easier and slightly\"I more accurate. So we are essentially measming the \"gauge\" pressure Pull out a presstne gauge and verify that the pressure at the top of the mob is indeed zero. This will be true for all of our uid measurements to follow. Pagez PHY213 Online Lab 07 3. For fun, briefly turn the Atmosphere back on. What is the Atmospheric pressure (in kPa) at the surface of the water? Atmospheric Pressure kPa 4. Turn the Atmosphere off again. Move the tip of the Fluid Density pressure gauge down to a depth of 2 meters. Put the value kom of the pressure in the table below. Put the value of the gasoline honey "Fluid Density" into the table as the Accepted Density for water. Fluid Density 5. Keeping the setup as it is, slide the Fluid Density to "gasoline." What is the gauge pressure now? Put that kg/m gasoline water honey value into the table. Put the Accepted Density for gasoline into the table as well. Fluid Density 6. Still keeping the same setup, now slide the Fluid Density to "honey." Record the pressure measurement at 2-meter ko/m depth in the table below. Also put the Accepted Density gasoline water honey for honey into the table. DENSITY Pressure at Calculated Accepted % error 2m depth Density Density Water Gasoline Honey Air Page 3PHY213 Online Lab 07 7. Our pressure measurements are the same as our change in pressure with depth, since we defined the top of the reservoir as being zero. Based on this, calculate the densities of water, gasoline, and honey respectively using the equation (with g = 9.80 m/s?): AP P= gAh 8. Put your calculated values into the table. (Realize that you will have to multiply by 1000 in order to convert kPa to Pascals.) How do your calculated values compare to the accepted values? 9. Air is a fluid too! Let's calculate the density of air. Click the Ruler 10 1 2 Atmosphere "On." Select the Ruler, and place the 1-meter Grid mark at the top of the fluid. Place the pressure gauge at the "0 m" mark on the ruler (see picture below). So there will be Atmosphere a "depth" of one meter between this pressure and the air On O Off pressure you measured earlier at the water surface. Find the AP in pressure. The Ah is one meter. Using the same equation as above, calculate the density of air. Compare this to the Accepted value of 1.225 kg/m'. Fill out the table. 10. Calculate the % errors between your calculated densities and the accepted values. Are these errors reasonable? Reminder: percent error = accepted value- experimental value -x100% accepted value Page 4PHY2I3 Online Lab 07 PART II: MYSTERY FLUIDS AND PLANETS 1. Select the bottom eongtmm'onthe one with the question mark '7". Turn off the Atmosphere. Turn on the Grid. Keep all other default values the same. Fill the whole reservoir by opening the inlet value. = Your screen should now look like: 2. With the atmosphere off, the atmospheric pressure at the top of the reservoir is zero. As you did with the the known fluick of water, gasoline, and honey above, nd the pressure at a 2-meter depth in Fluid A, Fluid B, and Fluid C respectively. 3. Now calculate the densities of the three mystery uids using the following equation (with g - 9.30 Im's'): p _ AP gAh 4. Assume that the mystery uids are from the following list. Identify the uids and their put their names in the table on the next page. PHY213 Online Lab 07 MYSTERY FLUIDS Pressure at Calculated Name 2m depth Density Fluid A Fluid B Fluid C 5. Now click on "Mystery Planet." Keep water as the fluid, and measure the pressure at a 2-meter depth. O Mystery Fluid Rearranging the above equation, the gravity of the planet Mystery Planet should be AP g planet = pAh 6. Fill in the values of the following table. MYSTERY PLANETS Pressure at Calculated 2m depth "g" Planet A Planet B Planet C Page 6PHYZB Online Lab 07 PART III: PRESSURE WlTH DEPTH AND PASCAL'S LAW 1. Click on the 3" conguration and reset El \"Burn the Atmosphere \"Off\PHY213 Online Lab 07 5. Keep the pressure gauges where they are. Click on the 500 kg mass, drag and place it in the left hand tube: Pressure Pressure Pressure kPa kPa kPa 3 m 6. What happens to the pressure readings? How much has the pressure increased? 7. After the 500 kg mass was positioned, what happened to the water level in the right-hand reservoir? Why do you think this happened? 8. Try applying more pressure with each 250 kg mass. What happens to the pressure readings now? How does this relate to Pascal's Law (see lab introduction)? Page 8PHY213 Online Lab 07 QUESTIONS 1. It is estimated that every 10 meters of depth in sea water adds about one atmosphere of pressure. You might be able to verify this using your earlier pressure measurements in pure water. But if not, try this: Using Configuration #1 with the Units set on "Atmospheres", and the Fluid Density set to seawater (1030 kg/m'), what do you observe Orwily when you measure the pressure at depths of Im, 2m, and 3m? 2. A diver swims into an underwater tunnel, with the intent to escape the extreme pressure of the sea. Will this strategy be successful? Why or why not? 3. Pascal's Law states that a pressure applied to an enclosed fluid is transferred undiminished throughout the fluid. We saw evidence of this when we placed the 500 kg mass on the surface of the water, and then observed what happened with the three pressure gauges. Suppose that the 500 kg mass just barely fit within the cylindrical shaft at the left. Based on your data, are you able to calculate the radius of the cylindrical shaft? Page 9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


