Question: please help me with this. i need the correct answers please !!!! Beneath the Things We See 25 Name: PRE-LAB ASSIGNMENT (Due before you start
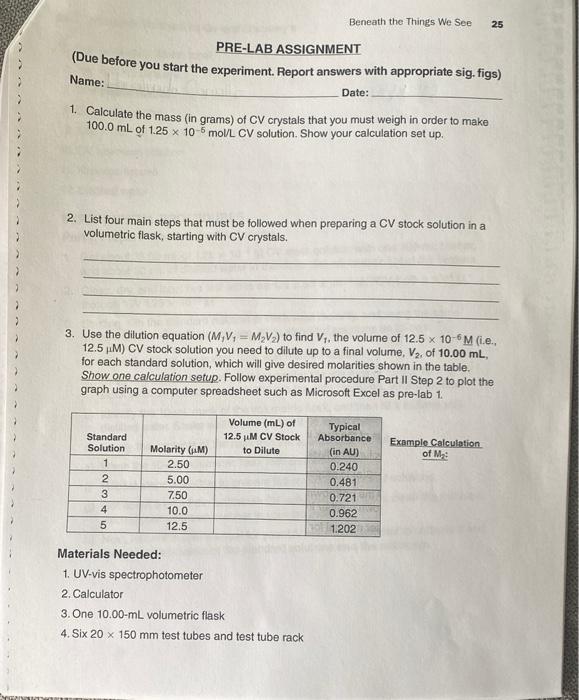
Beneath the Things We See 25 Name: PRE-LAB ASSIGNMENT (Due before you start the experiment. Report answers with appropriate sig. figs) Date: 1. Calculate the mass (in grams) of CV crystals that you must weigh in order to make 100.0 mL of 1.25 x 10 moul CV solution. Show your calculation set up. 2. List four main steps that must be followed when preparing a CV stock solution in a volumetric flask, starting with CV crystals. 3. Use the dilution equation (M, V = MV) to find V;. the volume of 12.5 x 10-6M (ie.. 12.5 M) CV stock solution you need to dilute up to a final volume, V2, of 10.00 mL, for each standard solution, which will give desired molarities shown in the table. Show one calculation setup. Follow experimental procedure Part II Step 2 to plot the graph using a computer spreadsheet such as Microsoft Excel as pre-lab 1. Standard Solution Volume (mL) of 12.5 M CV Stock to Dilute Example Calculation Typical Absorbance (in AU) 0.240 0.481 of M 2 3 4 5 AWN Molarity (M) 2.50 5.00 7.50 10.0 12.5 0.721 0.962 1.202 Materials Needed: 1. UV-vis spectrophotometer 2. Calculator 3. One 10.00-mL volumetric flask 4. Six 20 x 150 mm test tubes and test tube rack
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


