Question: Please help me with this if you know how to solve it. The final answer should contain only {Z, A, B} where Z=PV/RT, the compressibility
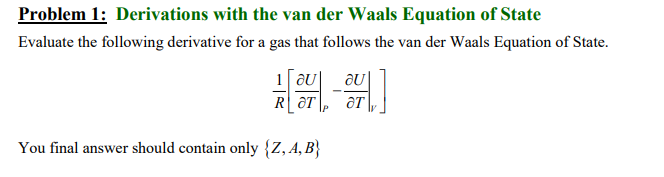
Please help me with this if you know how to solve it. The final answer should contain only {Z, A, B} where Z=PV/RT, the compressibility factor for ideal gas. A=27Pr/64Tr^2 and B= 1Pr/8Tr
where Pr is reduce pressure and Tr is reduce temperature. I will definitely rate your work. But please do not solve it if your answer doesn't contain only Z, A, B. Thank you.
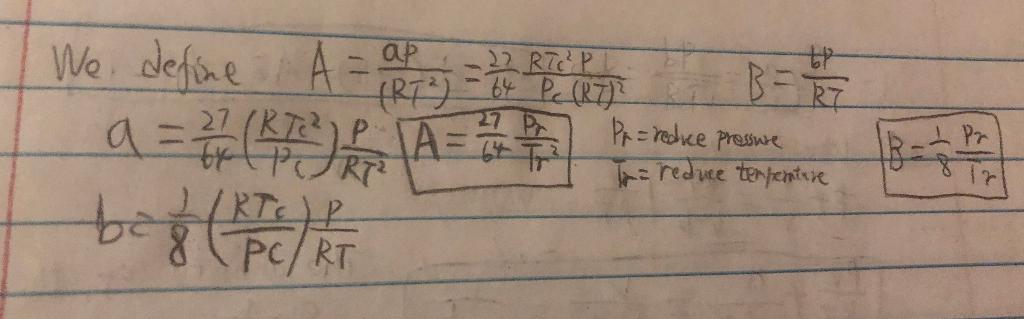
Problem 1: Derivations with the van der Waals Equation of State Evaluate the following derivative for a gas that follows the van der Waals Equation of State. 1 aula RaT ] You final answer should contain only {Z, A,B} 27 Pr 27 (RT P 1A=5+ To ap 23RTcP RT) - 64 Pe (R7? B = R7 Pr=redice pressure We define A = a 2 = 1 4 Reck) q=#PURE A beg 8 (PC/RT br LIRTO The reduce terfenture
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


