Question: Please help me with this problem I am stuck 25 points a) Calculate the work done by an ideal monoatomic gas during the expansion from
Please help me with this problem I am stuck
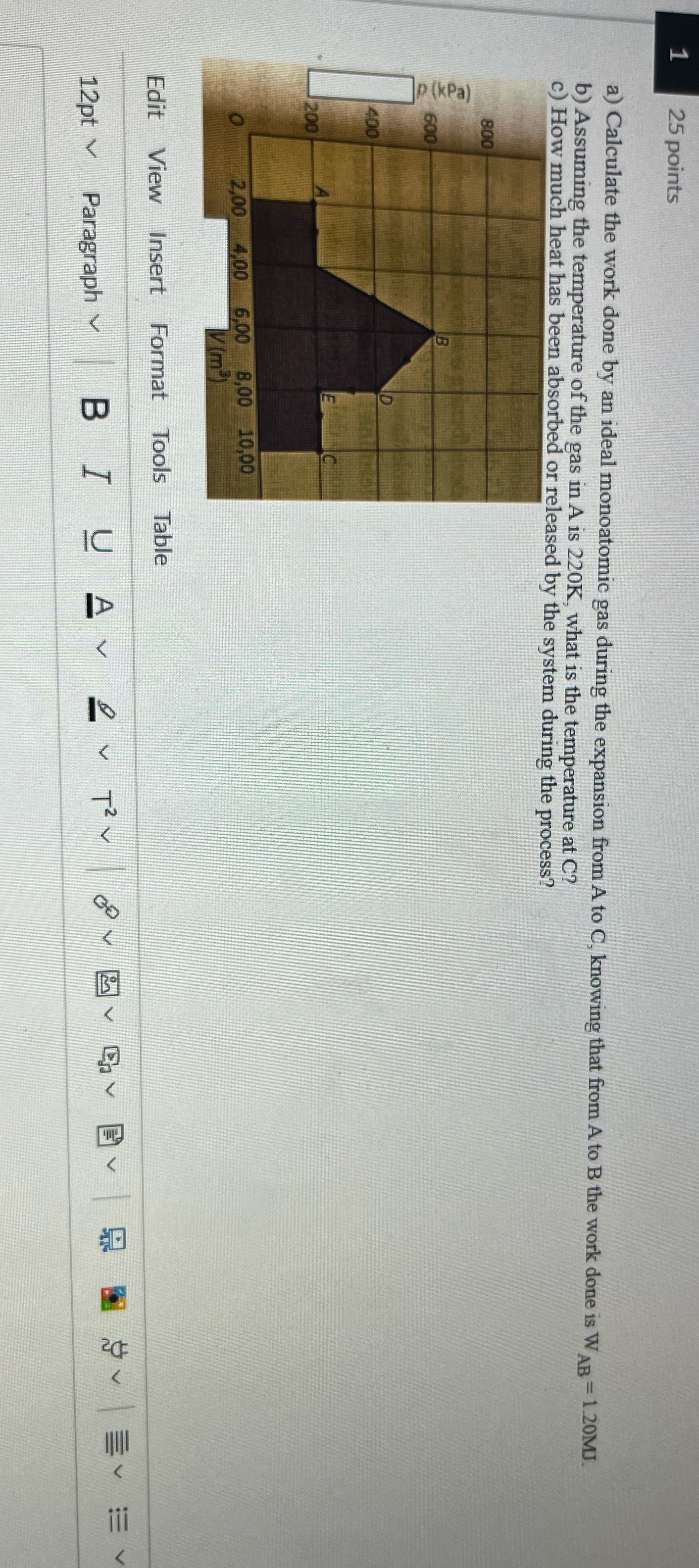
25 points a) Calculate the work done by an ideal monoatomic gas during the expansion from A to C, knowing that from A to B the work done is WAR = 1.20MJ b) Assuming the temperature of the gas in A is 220K, what is the temperature at C? c) How much heat has been absorbed or released by the system during the process? 800 P (kPa) 600 400 200 2,00 4,00 6,00 8,00 10,00 O V(m) Edit View Insert Format Tools Table V Ch v V SV EV EV Bo 12pt \\ Paragraph ~ B J U Av & v Ty
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


