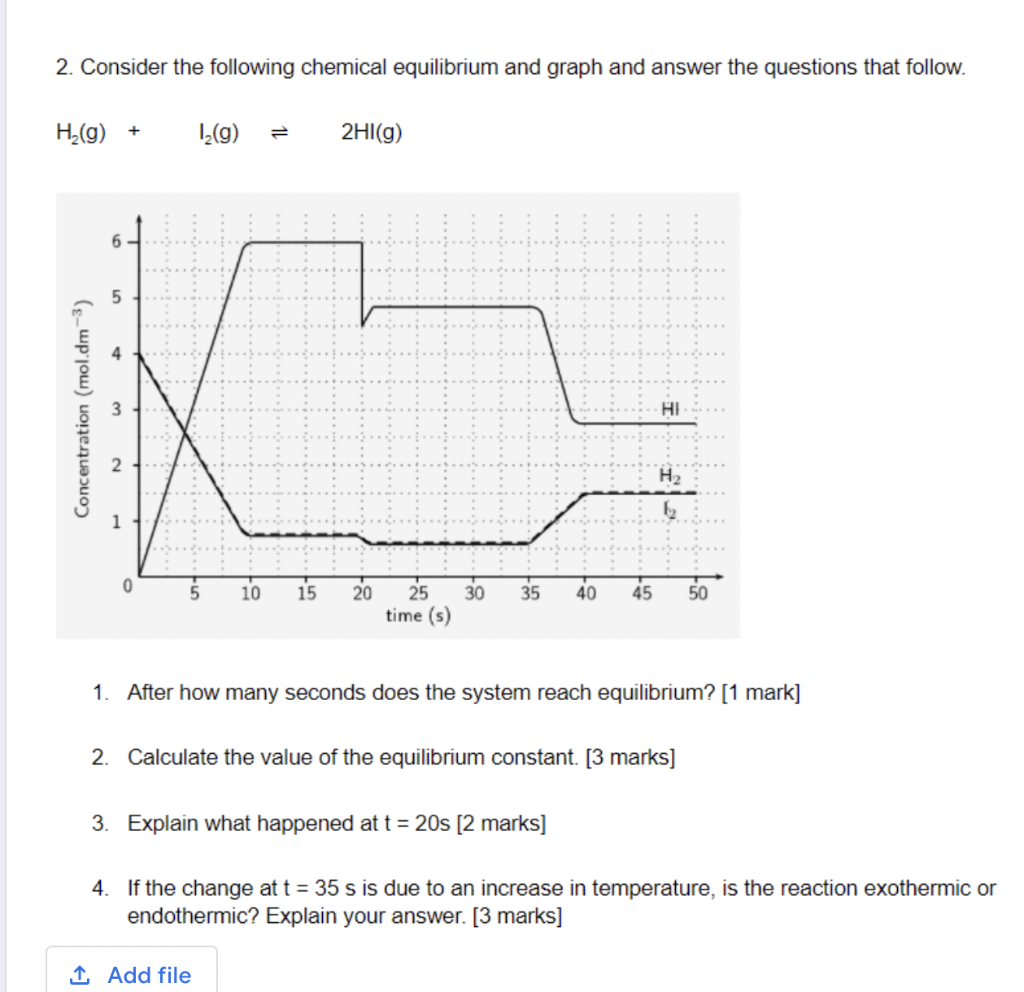
Question: Please help me with this question 2. Consider the following chemical equilibrium and graph and answer the questions that follow. H2(g) + 12(9) 2HI(g) 6
 Please help me with this question
Please help me with this question
2. Consider the following chemical equilibrium and graph and answer the questions that follow. H2(g) + 12(9) 2HI(g) 6 5 5 Concentration (mol.dm-3) 3 10 15 20 30 35 40 45 50 25 time (s) 1. After how many seconds does the system reach equilibrium? [1 mark] 2. Calculate the value of the equilibrium constant. [3 marks] 3. Explain what happened at t = 20s [2 marks] 4. If the change at t = 35 s is due to an increase in temperature, is the reaction exothermic or endothermic? Explain your answer. [3 marks] 1 Add file
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


