Question: Please help!! Need help ASAP!! Q6, Q7 Q7 OPTIONS: 1. lower or higher 2. london disperion forces or hydrogen bonding 3. lower or higher 4.
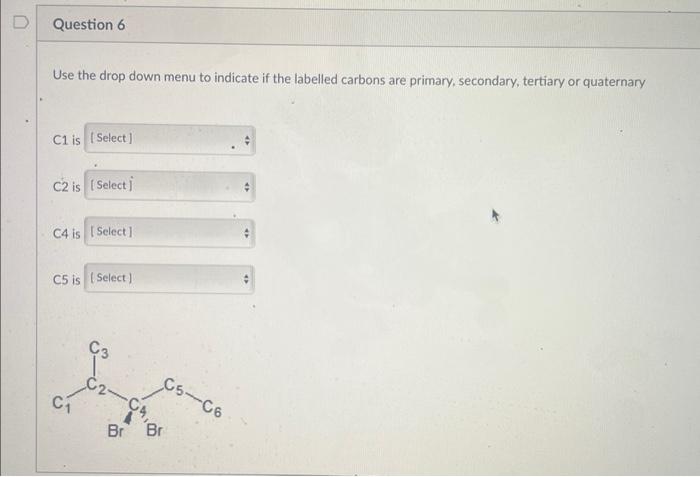
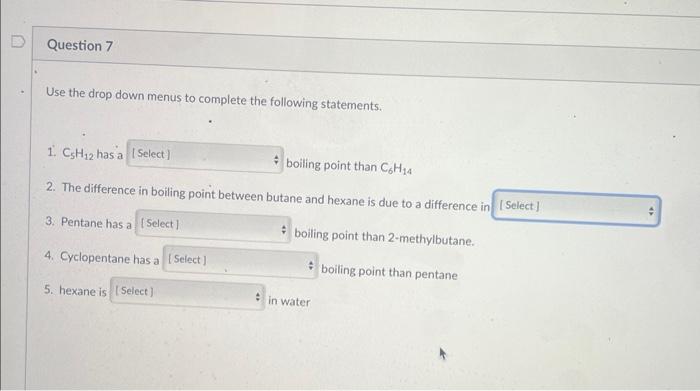
Use the drop down menu to indicate if the labelled carbons are primary, secondary, tertiary or quaternary C1 is C2 is C4 is C5 is Use the drop down menus to complete the following statements. 1. C5H12 hasa boiling point than C6H14 2. The difference in boiling point between butane and hexane is due to a difference in 3. Pentane has a boiling point than 2-methylbutane. 4. Cyclopentane has a boiling point than pentane 5. hexane is in water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


