Question: Please help on the blank ones and show work. thank you. will vote up !!!!!! Determining Reaction Orders Dl water 0.0720 M NaBro 0.180 M
Please help on the blank ones and show work. thank you. will vote up !!!!!!
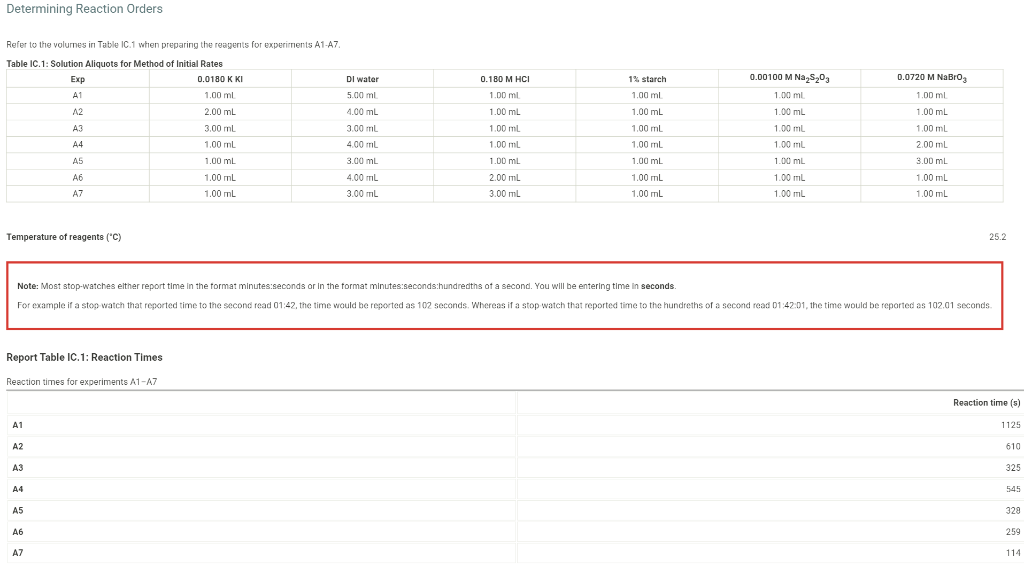
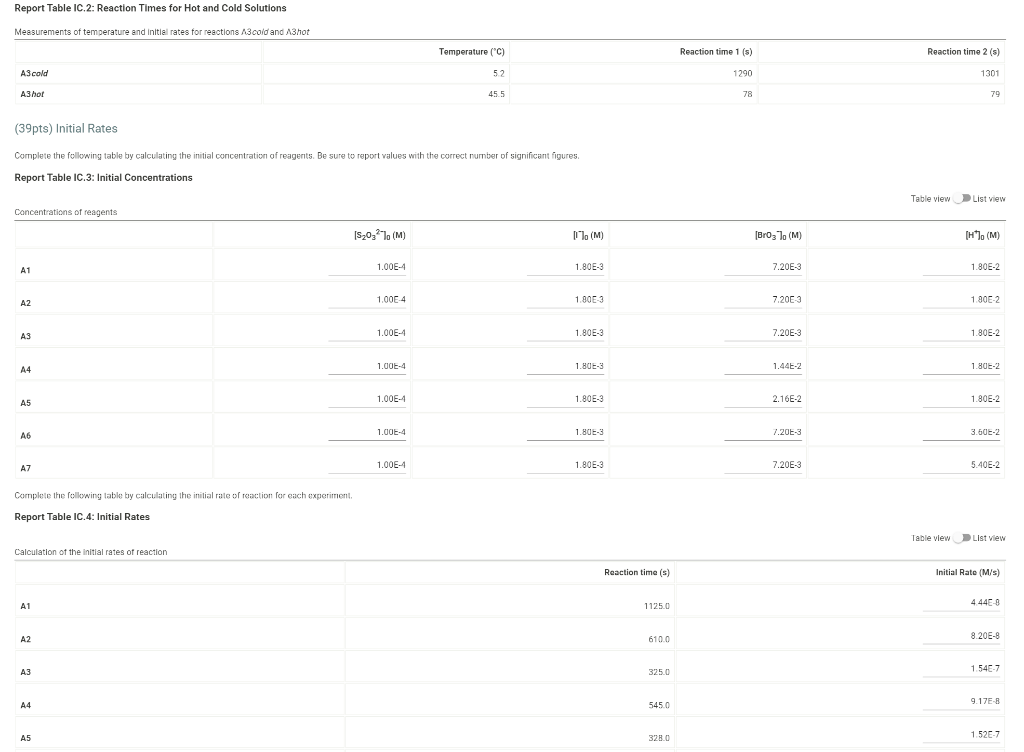
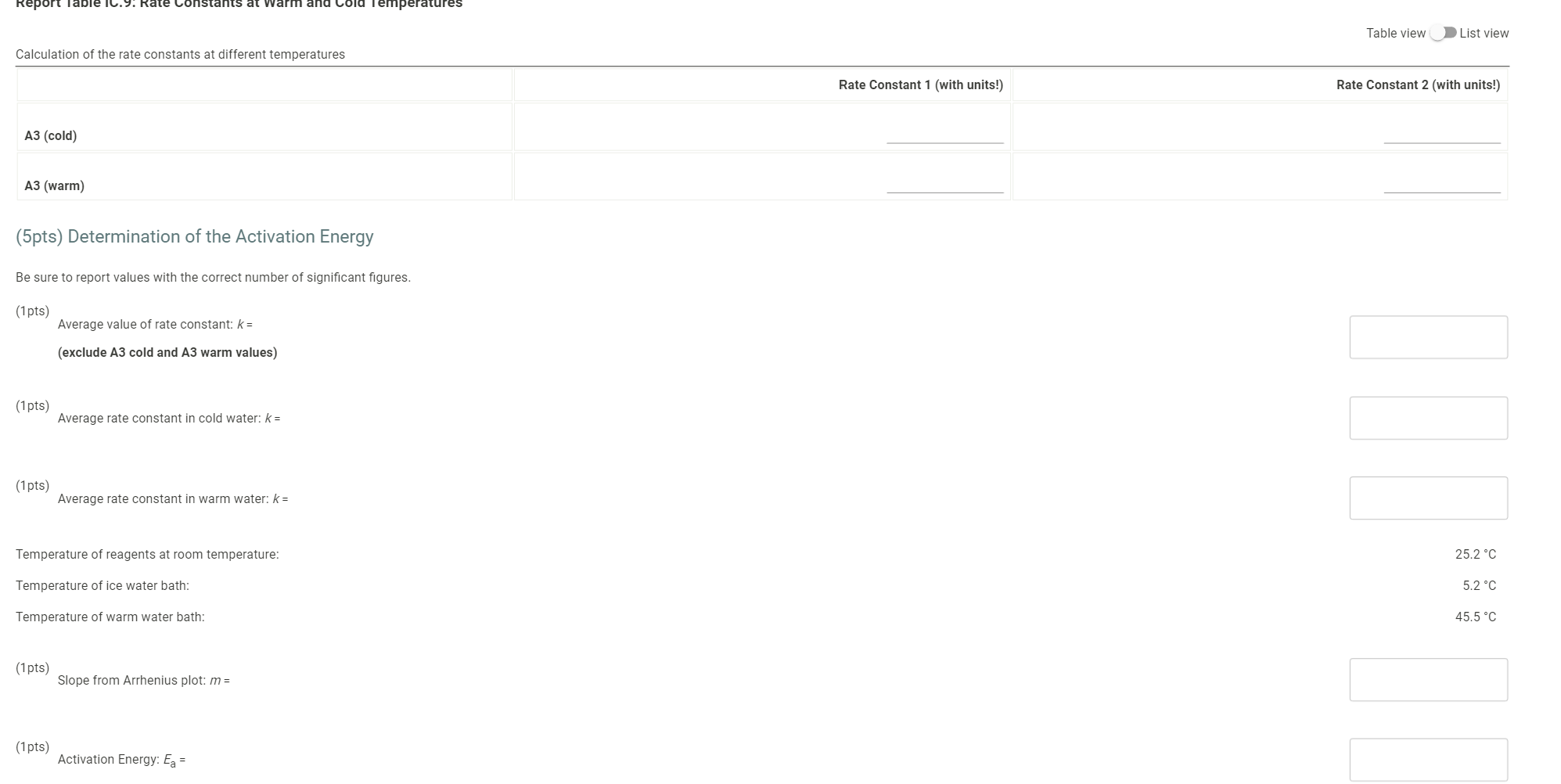
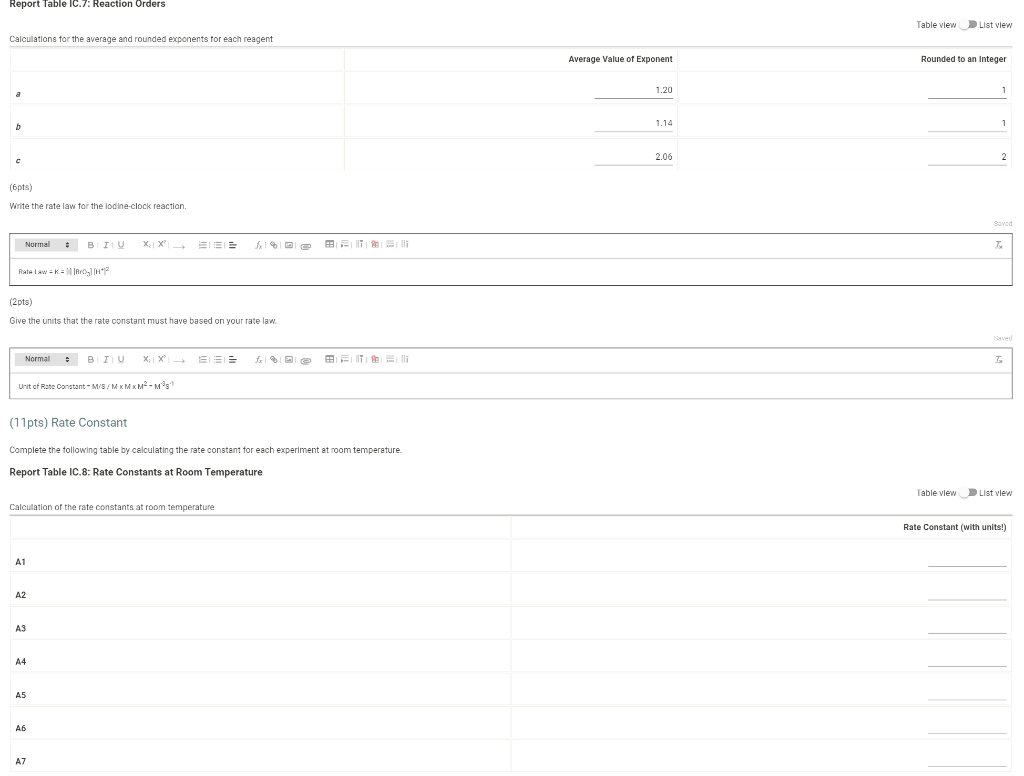
Determining Reaction Orders Dl water 0.0720 M NaBro 0.180 M HCI 1.00 ml 1% starch 1.00 ml 1.00 mL Refer to the volumnes in Table IC. 1 when preparing the reagents for experiments A1-A7 Table IC.1: Solution Aliquots for Method of Initial Rates Exp 0.0180 KKI A1 1.00 ml A2 2.00 mL A3 3.00 ml A4 14 1.00 mL A5 1.00 mL A6 1.00 mL A7 1.00 mL 5.00 ml 4.00 mL 3.00 ml 400 ml 3.00 mL 4.00 mL 3.00 ml 1.00 ml 0.00100 M Na 5,03 1.00 ml 1.00 mL 1.00 ml 1.00 ml 1.00 mL 1.00 mL 1.00 mL 1.00 ml 100 ml 1.00 mL 1.00 mL 1.00 ml 1.00 mL 1.00 ml 2.00 mL 3.00 mL 1.00 mL 1.00 ML 2.00 mL 3.00 mL 1.00 ml 1.00 mL 1.00 mL 1.00 mL Temperature of reagents ("C) 25.2 Note: Most stop-watches either report time in the format minutes:seconds or in the format minutes:seconds:hundredths of a second. You will be entering time in seconds For example if a stop watch that reported time to the second read 01:42, the time would be reported as 102 seconds. Whereas if a stop watch that reported time to the hundreths of a second read 01:42:01, the time would be reported as 102.01 seconds Report Table IC.1: Reaction Times Reaction times for experiments A1-A7 Reaction time (s) A1 1125 A2 610 A3 325 A4 545 A5 329 A6 259 AZ 114 Report Table IC.2: Reaction Times for Hot and Cold Solutions Measurements of temperature and initial rates for reactions A3 cold and Azhot Temperature (C) Reaction time 1 (s) Reaction time 2 (s) A3 cold 52 1290 1301 A3 hor 45.5 78 179 (39pts) Initial Rates Complete the following table by calculating the initial concentration of reagents. Be sure to report values with the correct number of significant figures. Report Table IC.3: Initial Concentrations Table view List View Concentrations of reagents IS2032 1. (M) lo (M) (Broz T. (M) [H]. (M) A1 1.00E-4 1.80E-3 7.20E-3 1.80E-2 A2 1.00E-4 1.80E-3 7.2013 1.80E-2 AS 1.00E-4 1.80E-3 7.20E-3 1.80E-2 A4 1.00E-4 1.80E 1.44E-2 1.80E-2 1.00E-4 1.80E-3 A5 2.16E-2 1.80E-2 A6 1.000-4 1.80E-3 7.2013 3.50E-2 A7 1.00E-4 1.80E-3 7.20E-3 5.40E-2 Complete the following table buy calculating the initial rate of reaction for each experiment Report Table IC.4: Initial Rates Table view List View Calculation of the initial rates of reaction Reaction time (s) Initial Rate (M/s) A1 11250 444E-8 A2 6100 8.20E-8 A3 325.0 1.54E-7 A4 5450 9.17E 8 A5 328.0 1.52E-7 Report Table ic.9. Rate Constants at Warm and old Temperatures Table view List view Calculation of the rate constants at different temperatures Rate Constant 1 (with units!) Rate Constant 2 (with units!) A3 (cold) A3 (warm) (5pts) Determination of the Activation Energy Be sure to report values with the correct number of significant figures. (1 pts) Average value of rate constant: k = (exclude A3 cold and A3 warm values) (1 pts) Average rate constant in cold water: k = (1 pts) Average rate constant in warm water: k = Temperature of reagents at room temperature: 25.2 C Temperature of ice water bath: 5.2 C Temperature of warm water bath: 45.5 C (1 pts) Slope from Arrhenius plot: m = (1pts) Activation Energy: Ea = Report Table Ic.7: Reaction Orders Table view List view Calculations for the average and rounded exponents for each reagent Average Value of Exponent Rounded to an Integer 1.20 a 1 1 1.14 1 b 2.06 c 2 (6 pts) Write the rate law for the lodine-clock reaction. saved Normal + BIU EEE Billi 7 Pirate law = K = M** = = 12pts) Give the units that the rate constant must have based on your rate law. Normal XXE BT lli Unit of Rate Constant - M/S/MKMXM-M1 (11 pts) Rate Constant Complete the following table by calculating the rate constant for each experiment at room temperature, Report Table IC.8: Rate Constants at Room Temperature Table view List view Calculation of the rate constants at room temperature Rate Constant (with units!) A1 A2 AS A4 A5 A6 A7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


