Question: please help! please list correct option for last problem(ex:1,2,3) thank you so much! 12.11a2 Get help answering Molecular Drawing questions, Provide the missing curved arrow(s)
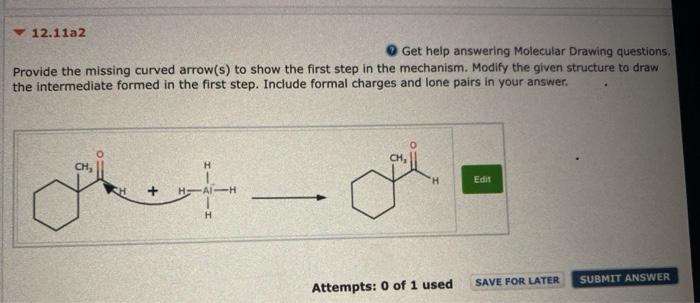
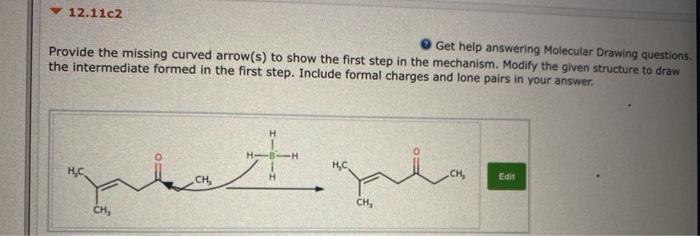

12.11a2 Get help answering Molecular Drawing questions, Provide the missing curved arrow(s) to show the first step in the mechanism. Modify the given structure to draw the intermediate formed in the first step. Include formal charges and lone pairs in your answer. CH, H Edit HAH H SAVE FOR LATER SUBMIT ANSWER Attempts: 0 of 1 used 12.11c2 Get help answering Molecular Drawing questions Provide the missing curved arrow(s) to show the first step in the mechanism. Modify the given structure to draw the intermediate formed in the first step. Include formal charges and lone pairs in your answer. H H, HC CW Edit CH, CH, Question 5 In Chapter 8, we learned about addition of water across a pi bond. Identify whether the alkene has been oxidized, reduced, or neither. (Hint: First look at each carbon atom separately and then look at the net change for the alkene as a whole.) Try to determine the answer without calculating oxidation states and then use the calculations to see if your intuition was correct. H OH H30* The first carbon atom (with added H) has been oxidized and the second carbon atom (with added OH) has also been oxidized. Overall, the alkene has been oxidized. The first carbon atom (with added H) has been reduced and the second carbon atom (with added OH) has also been reduced. Overall, the alkene has been reduced. The first carbon atom (with added H) has been reduced and the second carbon atom (with added OH) has been oxidized. Overall, the alkene does not undergo a net change in oxidation state and is, therefore, neither reduced nor oxidized. The first carbon atom (with added H) has been oxidized and the second carbon atom (with added OH) has been reduced. Overall, the alkene does not undergo a net change in oxidation state and is, therefore, neither reduced nor oxidized
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


