Question: please help! Question 10 (1 point) A student measured qrn for the reaction of 0.468g of a metal, Xis (molar mass = 88.12 ), with
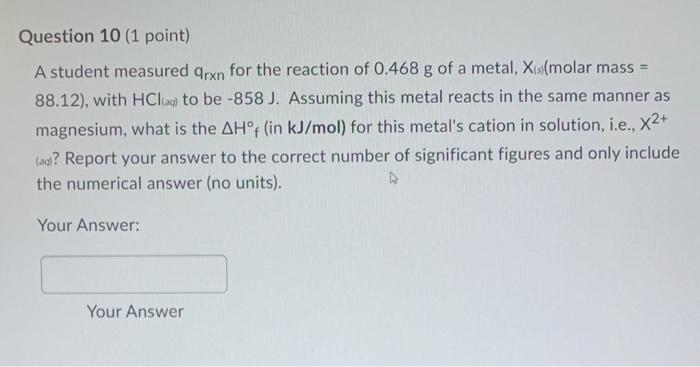
Question 10 (1 point) A student measured qrn for the reaction of 0.468g of a metal, Xis (molar mass = 88.12 ), with HCl(aq) to be 858J. Assuming this metal reacts in the same manner as magnesium, what is the Hf (in kJ/mol) for this metal's cation in solution, i.e., X2+ (aa)? Report your answer to the correct number of significant figures and only include the numerical answer (no units). Your Answer: Your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


