Question: Please help! Question 2. Often at large scale in industry, distillation can be combined during a chemical reaction to exploit chemical equilibrium. How might distillation
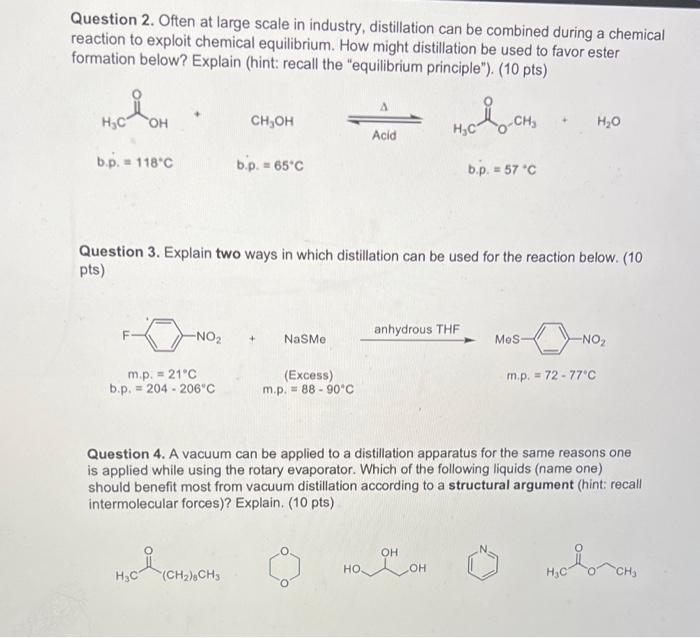
Question 2. Often at large scale in industry, distillation can be combined during a chemical reaction to exploit chemical equilibrium. How might distillation be used to favor ester formation below? Explain (hint: recall the "equilibrium principle"). (10 pts) CH3OH=Acid b. p.=118C b.p. =65C b.p. =57C Question 3. Explain two ways in which distillation can be used for the reaction below. (10 pts) Question 4. A vacuum can be applied to a distillation apparatus for the same reasons one is applied while using the rotary evaporator. Which of the following liquids (name one) should benefit most from vacuum distillation according to a structural argument (hint: recall intermolecular forces)? Explain. ( 10pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


