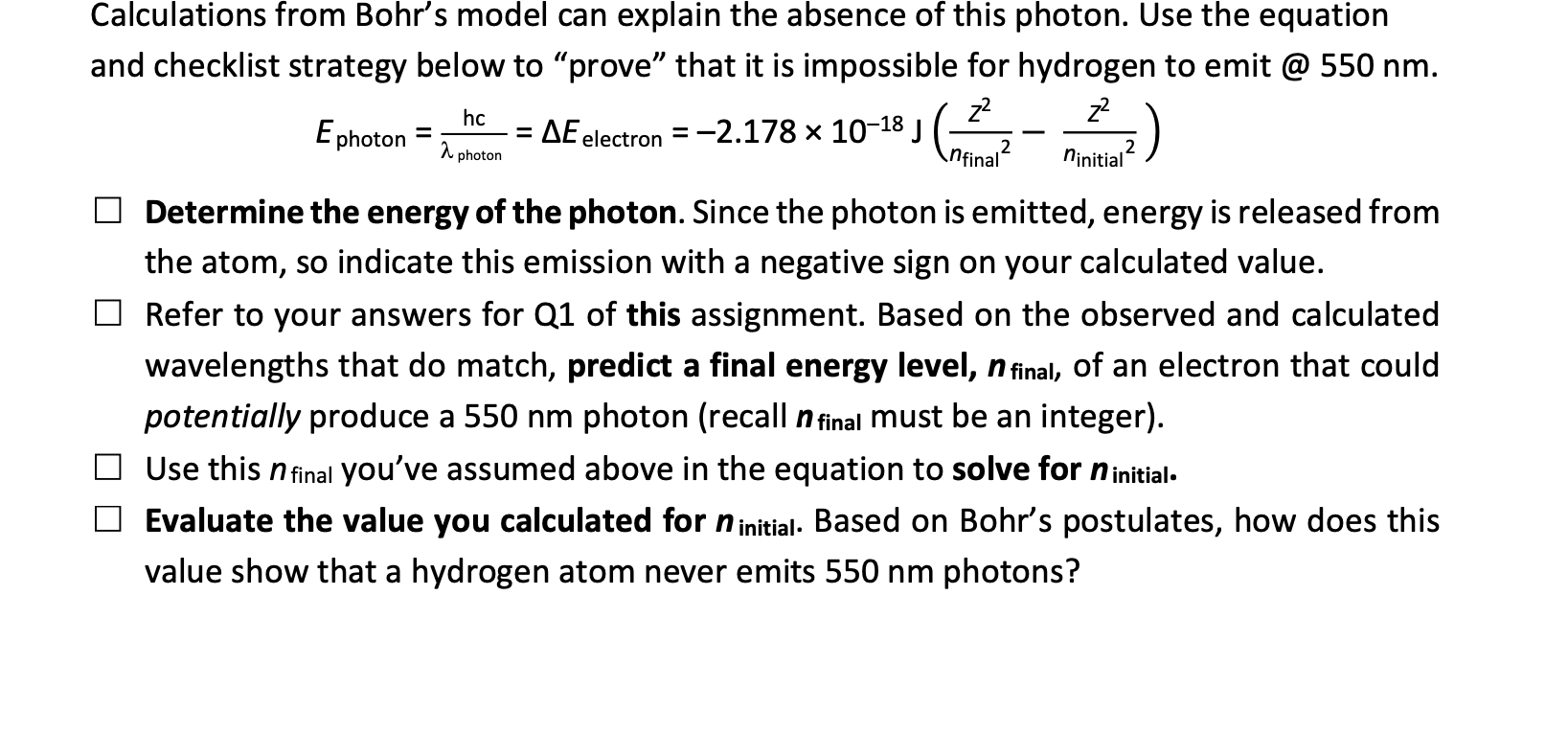
Question: please help quickly question 1: Calculations from Bohr's model can explain the absence of this photon. Use the equation and checklist strategy below to prove
please help quickly
question 1: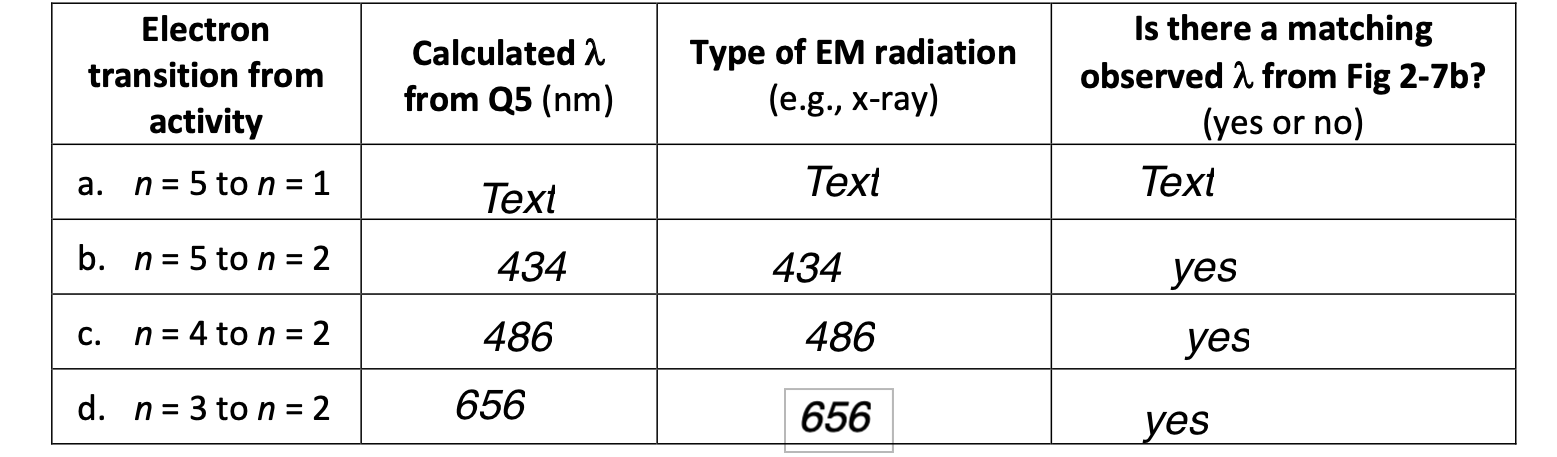
Calculations from Bohr's model can explain the absence of this photon. Use the equation and checklist strategy below to "prove" that it is impossible for hydrogen to emit @ 550nm. Ephoton=photonhc=Eelectron=2.1781018J(nfinal2z2ninitial2z2) Determine the energy of the photon. Since the photon is emitted, energy is released from the atom, so indicate this emission with a negative sign on your calculated value. Refer to your answers for Q1 of this assignment. Based on the observed and calculated wavelengths that do match, predict a final energy level, nfinal, of an electron that could potentially produce a 550nm photon (recall nfinal must be an integer). Use this nfinal you've assumed above in the equation to solve for ninitial.. Evaluate the value you calculated for ninitial. Based on Bohr's postulates, how does this value show that a hydrogen atom never emits 550nm photons? \begin{tabular}{|c|c|c|c|} \hline Electron transition from activity & Calculated from Q5 (nm) & Type of EM radiation (e.g., x-ray) & Is there a matching observed from Fig 2-7b? (yes or no) \\ \hline a. n=5 to n=1 & Text & Text & Text \\ \hline b. n=5 to n=2 & 434 & 434 & yes \\ \hline c. n=4 to n=2 & 486 & 486 & yes \\ \hline d. n=3 to n=2 & 656 & 656 & yes \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


