Question: PLEASE HELP Report Submission - Buffer Solutions (24pts) Buffers How will you collect data for this experiment? in person Preparation of Buffer Solutions Data Mass
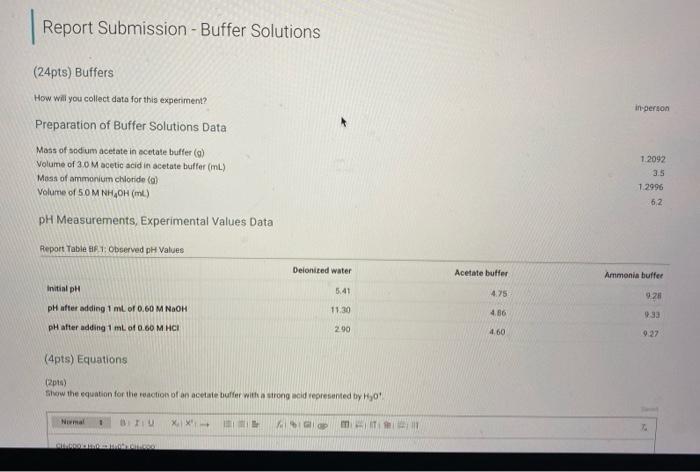
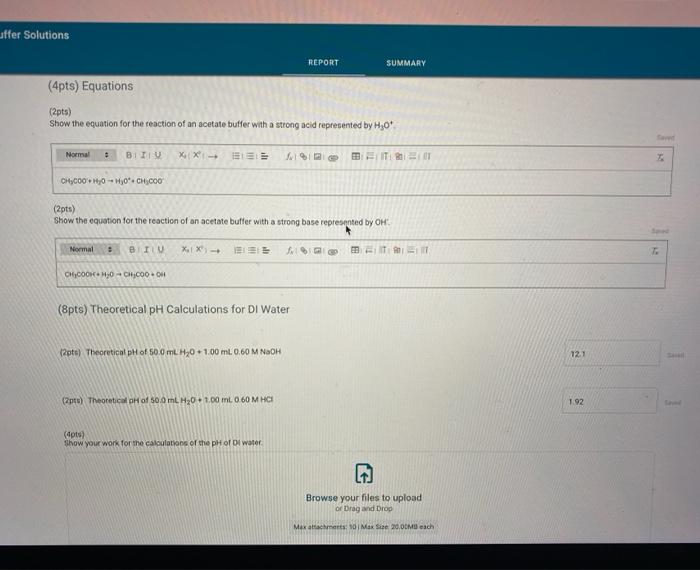
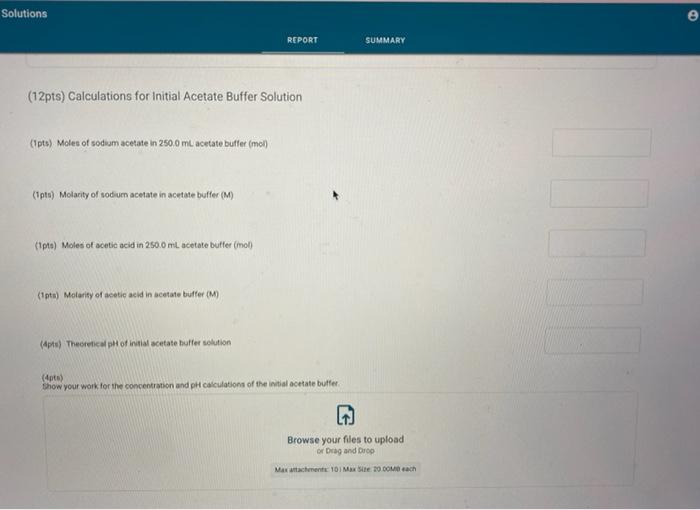
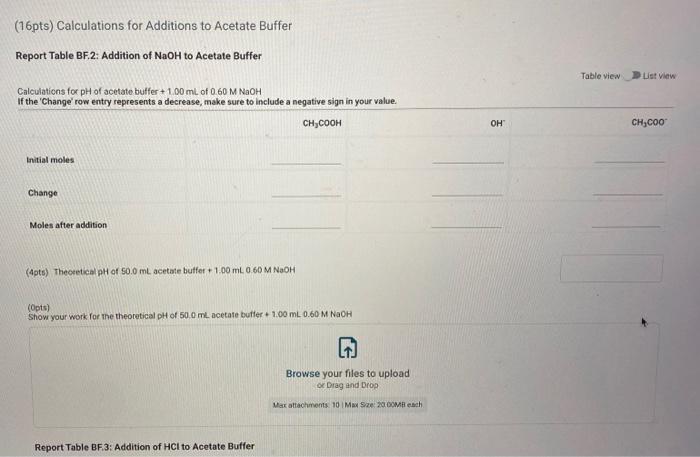
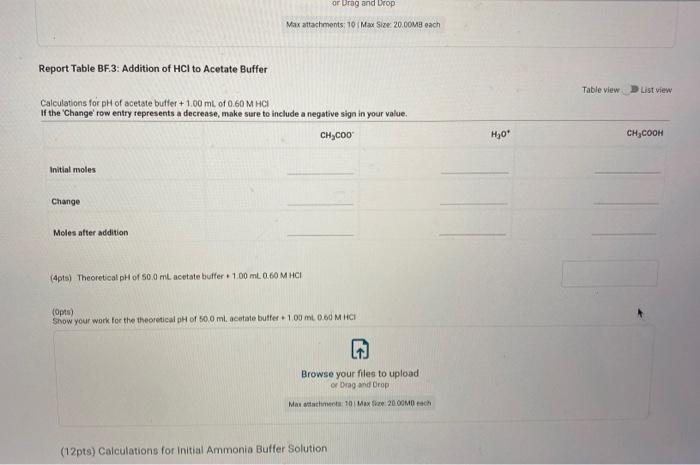
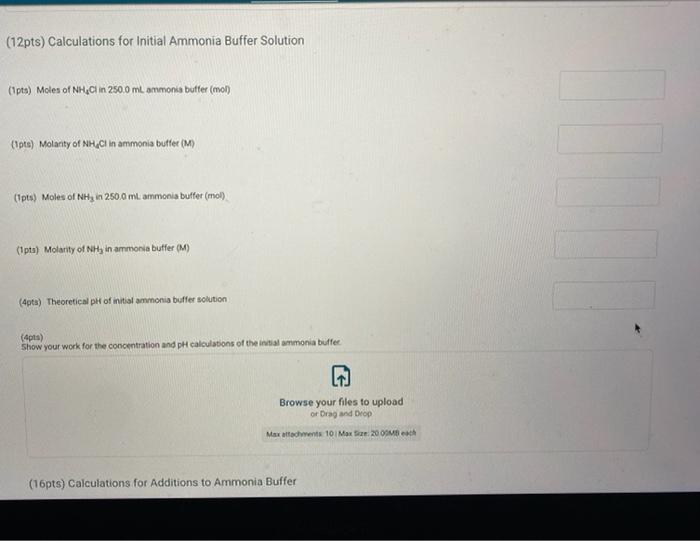
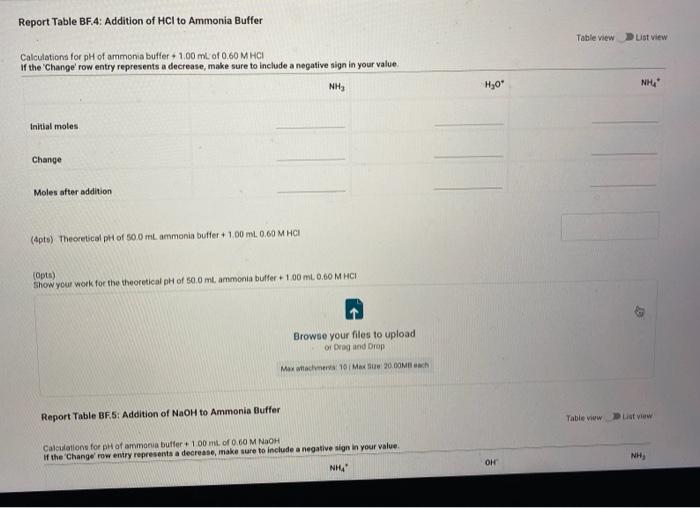
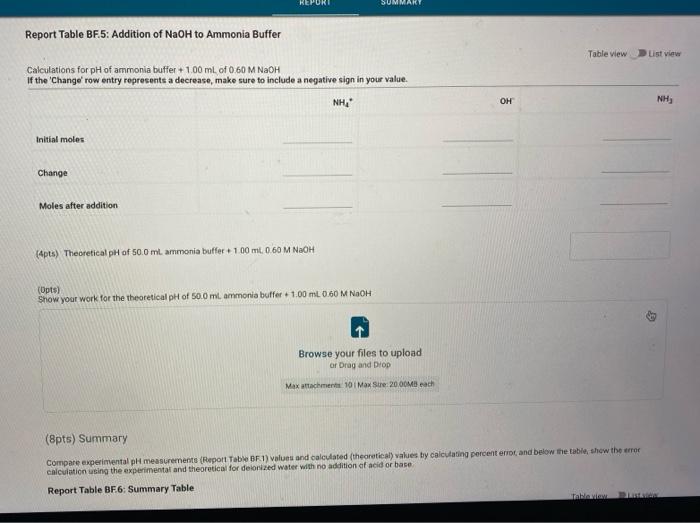
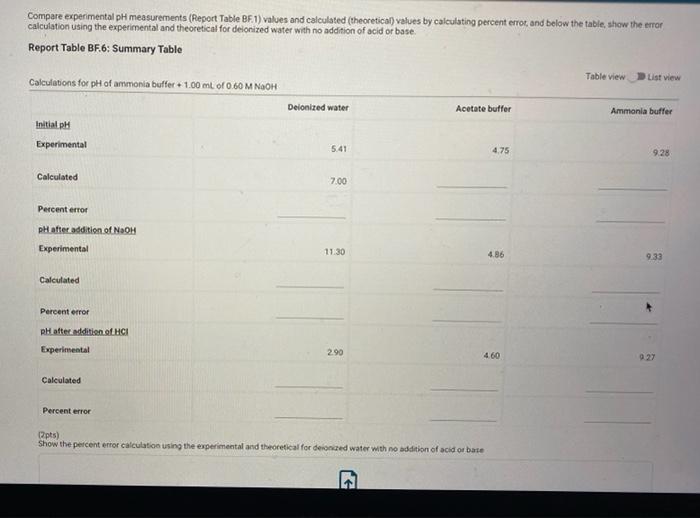
Report Submission - Buffer Solutions (24pts) Buffers How will you collect data for this experiment? in person Preparation of Buffer Solutions Data Mass of sodium acetate in acetate buffer (0) Volume of 3.0 Macetic acid in acetate buffer (ML) Moss of ammonium chloride (a) Volume of 50M NH,OH (ml) 1.2092 3.5 1 2996 62 PH Measurements, Experimental Values Data Report Table Observed pH values Delonited water Acetate buffer Armenia buffer Initial pH 5.41 4.75 9.28 pH after adding 1 ml of 0.60 M NAOH 11.30 4.86 33 pH after adding 1 mL of 0.60 M HCI 2.90 4.60 9.27 (4pts) Equations (pt) Show the equation for the reaction of an acetate buffer with a strong acid represented by Ho Normal Bri XX Go RURAL uffer Solutions REPORT SUMMARY (4pts) Equations (2pts) Show the equation for the reaction of an acetate buffer with a strong acid represented by H,09 Normal . BI ITU XX- ESIE BEITB CHCOOHO - HO CHICCO (2pts) Show the equation for the reaction of an acetate buffer with a strong base represented by OH Normal . BIU XIX- == BIT CCOOK+H30 CCOOOH (8pts) Theoretical pH Calculations for Di Water 2p) Theoretical pH of mL H20 +1.00 mL. 0.60 M NOCH 121 (2010) Theoretical of 50.0 mL M20 + 1,00 m. 0 60 M HCI 1.92 (dot) Show your work for the calculations of the pH of Diwater Browse your files to upload of Drag and drop Max attachments: 0 Max Size 20.00M each Solutions REPORT SUMMARY (12pts) Calculations for Initial Acetate Buffer Solution Cipta) Moles of sodium acetate in 250.0 mL acetate butter (mol) (Ita) Molacity of sodium acetate in acetate buffer (M) (pte) Moles of acetic acid in 250.0 ml acetate buffer (mol) (pt) Moterity of coetic and in acetate buffer (M) (pte) Theoretical pH of innocetate butter solution (406) Show your work for the concentration and pH calculation of the wil acetate buffer Browse your files to upload or Drag and drop Marche 10 Mate 10 Mach (16pts) Calculations for Additions to Acetate Buffer Report Table BF.2: Addition of NaOH to Acetate Buffer Table view Ust view Calculations for pH of acetate buffer +1.00 mL of 0.60 M NaOH If the 'Change row entry represents a decrease make sure to include a negative sign in your value. CH,COOH " CH,COo Initial moles Change Moles after addition (Apts) Theoretical pH of 500 ml acetate buffer +1.00 mL 0.60 M NaOH (Opts) Show your work Yor the theoretical pH of 50.0 ml acetate butter 100 ML 0.60 M NACH Browse your files to upload o Drag and Drop Max attachments 10 MS 2000M each Report Table BF3: Addition of HCl to Acetate Buffer or Drag and Drop Max attachments: 10 Max Size: 20.00MB cach Report Table BF.3: Addition of HCl to Acetate Buffer Table view List view Calculations for pH of acetate buffer +1.00 mL of 0.60 MHCI If the Change row entry represents a decrease, make sure to include a negative sign in your value. CH,COO * CH,COOH Initial moles Change Moles after addition (Apts) Theoretical pH of 500 ml acetate buffer 100 ml. 0.60 M HCI (Opts) Snow your work for the theoretical pH of 500 ml acetate butter + 100 m. 0.00 MHCI Browse your files to upload of Drag and Drop Man 10 Max 2000MD rach (12pts) Calculations for initial Ammonio Buffer Solution (12pts) Calculations for Initial Ammonia Buffer Solution (1 pts) Moles of NH4Cl in 250.0 ml ammonia buffer (mol) (pte) Molarity of NH CI in ammonia buffer (M) (1pts) Moles of NH, in 250.0 ml ammonia buffet (mol) (pta) Molarity of NH, in ammonia buffer (M) (4pta) Theoretical pH of initial ammonia buffer solution (4pts) Show your work for the concentration and pH calculations of the initial amonia buffee Browse your files to upload or Drag and drop Max attedwants 10 Max fie 2008 (16pts) Calculations for Additions to Ammonia Buffer Report Table Bf.4: Addition of HCl to Ammonia Buffer Table view List View Calculations for pH of ammonia buffer +1.00 ml of 0,60 M HCI If the 'Change' row entry represents a decrease, make sure to include a negative sign in your value NH HO NHA Initial moles Change Moles after addition (Apts) Theoretical pH of 500 ml ammonia buffer + 100 ML.0.60 M HCI (Opt) Show your work for the theoretical pH of 500 m, ammonia buffer +1.00 mt. 0.60 MHCI Browse your files to upload or Drag and drop Max with 10 Max 20.00MB each Report Table BF 5: Addition of NaOH to Ammonia Buffer Table view List View Calculation for pH of ammonia buffer +1.00 mL of 0.60 M NOOH If the 'Change row entry represents a decrease make sure to include a negative sign in your value. NH OH NHA KEPORI SUMMAR Report Table BF.5: Addition of NaOH to Ammonia Buffer Table view List View Calculations for pH of ammonia buffer + 100 ml of 0.60 M NaOH If the 'Change' row entry represents a decrease, make sure to include a negative sign in your value. NH, OH NH, Initial moles Change Moles after addition (Apts) Theoretical pH of 50.0 ml ammonia buffer + 100 ml 0.60 M NaOH (Opts) Show your work for the theoretical pH of 50.0 ml ammonia buffer 1.00 mL 0.60 M NOCH + Browse your files to upload or Drag and Drop Max attachment 10 Max Ste 2000MB each (pts) Summary Compare experimental pH measurements (Report Table 1) values and calculated (theoretical values by calculating percent error and below the table, show the error calculation using the experimental and theoretical for delonized water with no addition of acid or base Report Table BF 6: Summary Table Table Compare experimental pH measurements (Report Table BF 1) values and calculated (theoretical) values by calculating percent error, and below the table, show the error calculation using the experimental and theoretical for deionized water with no addition of acid or base Report Table BF.6: Summary Table Table view Calculations for pH of ammonia buffer + 1.00 m of 0.60 M NACH Acetate buffer List View Deionized water Ammonia buffer Initial pH Experimental 5.41 4.75 9.28 Calculated 7.00 Percent error pH after addition of NOOH Experimental 11.30 4.86 9.33 Calculated Percent error RH after addition of HCI Experimental 290 4.60 0.27 Calculated Percent error 2pts) Show the percent error calculation using the experimental and theoretical for deinced water with no addition of acid or bate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


