Question: Please help solve for these three. If u answer fast i will give thumbs up 2 A + B + 2 C2 E + 3
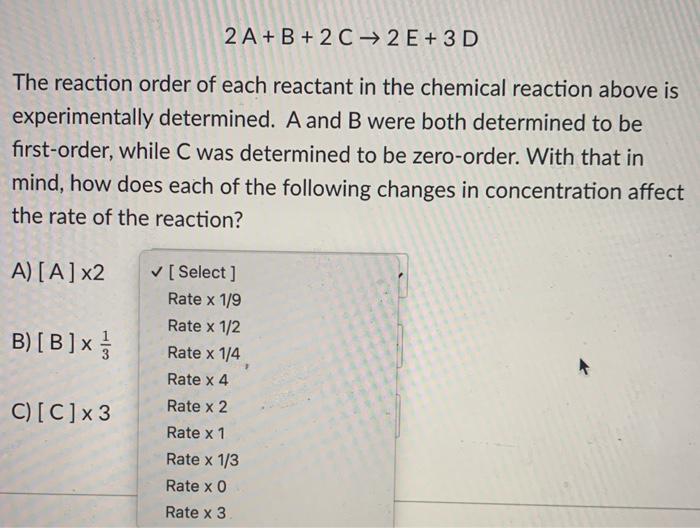
2 A + B + 2 C2 E + 3 D The reaction order of each reactant in the chemical reaction above is experimentally determined. A and B were both determined to be first-order, while C was determined to be zero-order. With that in mind, how does each of the following changes in concentration affect the rate of the reaction? A) [A] x2 B) [B]x [ Select ] Rate x 1/9 Rate x 1/2 Rate x 1/4 Rate x 4 Rate x 2 Rate x1 Rate x 1/3 Rate x 0 Rate x3 C)[C]x3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


