Question: Please help solving these 3 problems sorry it's only 2 questions National Ambient Air Quality Standards set the 8-hour ozone exposure level (the maximum concentration


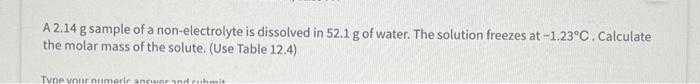
National Ambient Air Quality Standards set the 8-hour ozone exposure level (the maximum concentration to which you can safely be exposed to during an 8 -hour period) at 0.0700 ppmv. If you're working in a room containing 1114L of air at STP for an eight-hour period, then what is the maximum volume of ozone in L allowed in the room? Answer using E notation. A 2.14g sample of a non-electrolyte is dissolved in 52.1g of water. The solution freezes at 1.23C. Calculate the molar mass of the solute. (Use Table 12.4) A 2.14g sample of a non-electrolyte is dissolved in 52.1g of water. The solution freezes at 1.23C. Calculate the molar mass of the solute. (Use Table 12.4)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


