Question: please help solving, those picture are nit test/exam/ or quiz content Molecular formula: (5) Lewis Structure: (5) No. of valence electrons: (5) Molecular weight: (5)
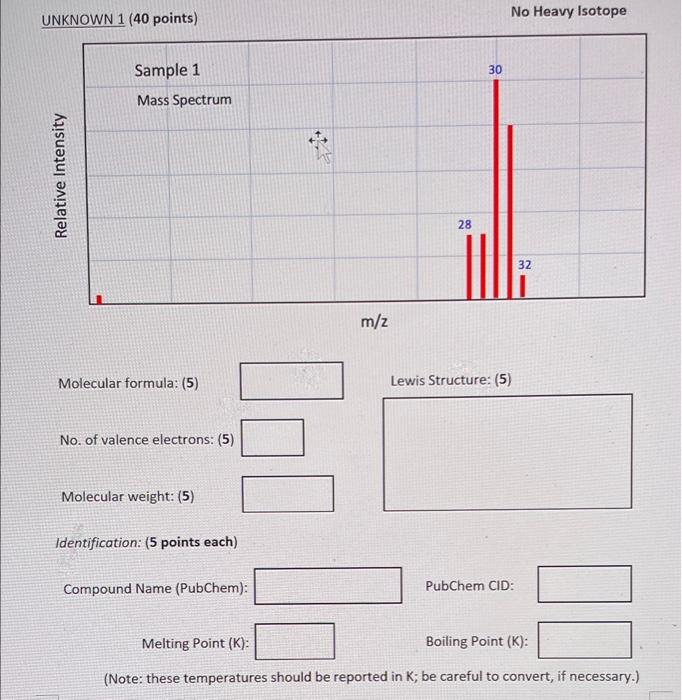
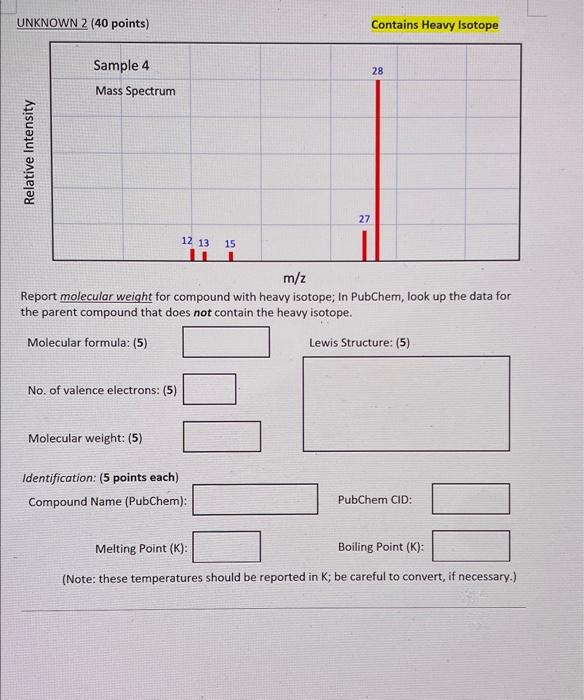
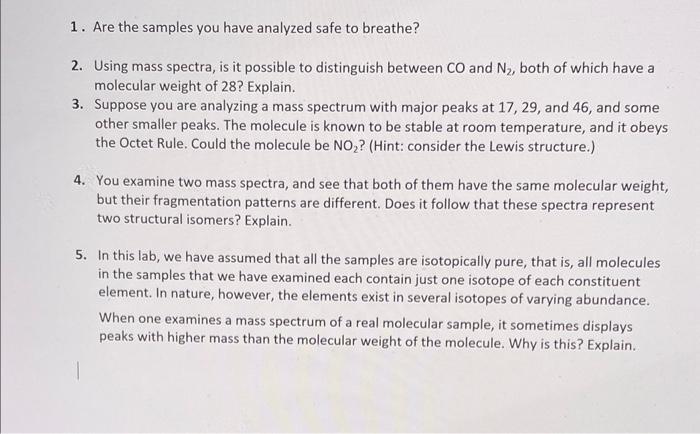
Molecular formula: (5) Lewis Structure: (5) No. of valence electrons: (5) Molecular weight: (5) Identification: (5 points each) Compound Name (PubChem): PubChem CID: Melting Point ( K : Boiling Point ( K ): (Note: these temperatures should be reported in K; be careful to convert, if necessary.) UNKNOWN 2 ( 40 points) Contains Heavy Isotope Report molecular weight for compound with heavy isotope; In PubChem, look up the data for the parent compound that does not contain the heavy isotope. Molecular formula: (5) Lewis Structure: (5) No. of valence electrons: (5) Molecular weight: (5) Identification: (5 points each) Compound Name (PubChem): PubChem CID: Melting Point ( K ) : Boiling Point (K): (Note: these temperatures should be reported in K; be careful to convert, if necessary.) 1. Are the samples you have analyzed safe to breathe? 2. Using mass spectra, is it possible to distinguish between CO and N2, both of which have a molecular weight of 28 ? Explain. 3. Suppose you are analyzing a mass spectrum with major peaks at 17,29 , and 46 , and some other smaller peaks. The molecule is known to be stable at room temperature, and it obeys the Octet Rule. Could the molecule be NO2 ? (Hint: consider the Lewis structure.) 4. You examine two mass spectra, and see that both of them have the same molecular weight, but their fragmentation patterns are different. Does it follow that these spectra represent two structural isomers? Explain. 5. In this lab, we have assumed that all the samples are isotopically pure, that is, all molecules in the samples that we have examined each contain just one isotope of each constituent element. In nature, however, the elements exist in several isotopes of varying abundance. When one examines a mass spectrum of a real molecular sample, it sometimes displays peaks with higher mass than the molecular weight of the molecule. Why is this? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


