Question: please help, thank you (25 points) You have a mixture of dimethylbubblegum (1) and diethlybubblegum (2) that is 25 mole percent dimethylbubblegum (1). For each
please help, thank you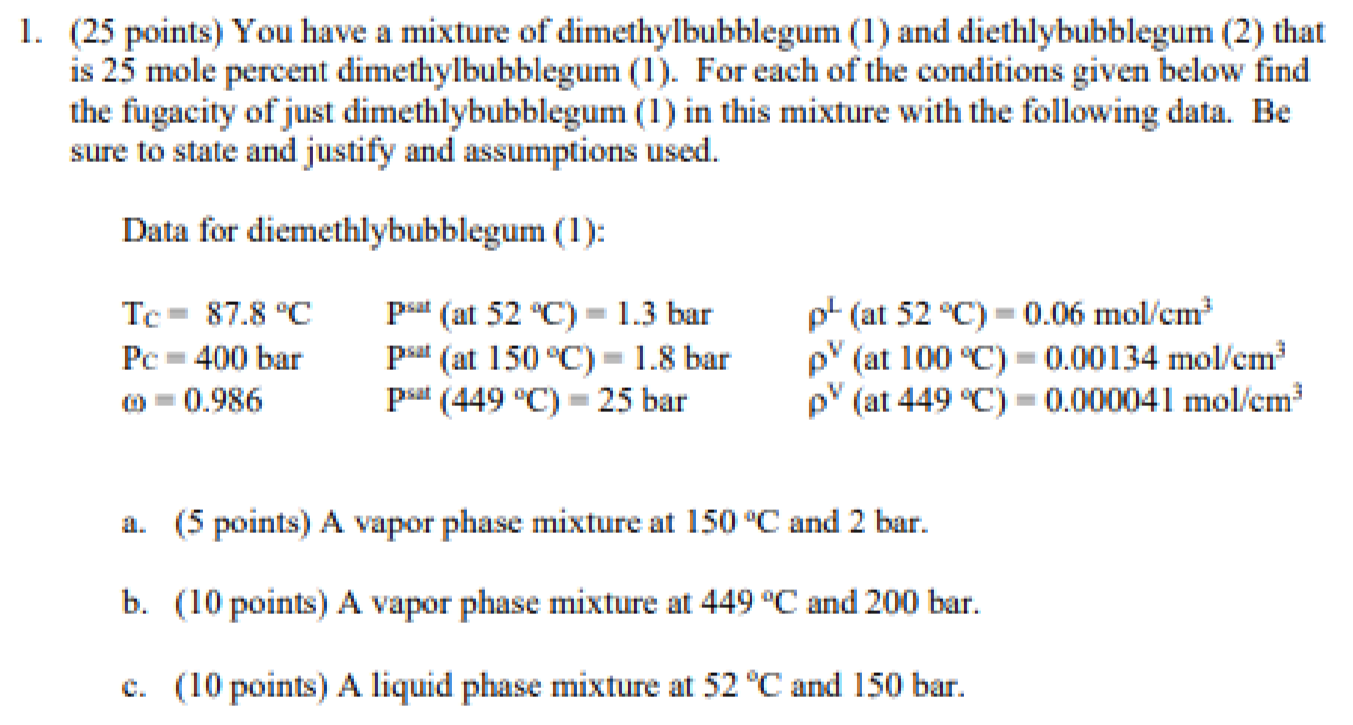
(25 points) You have a mixture of dimethylbubblegum (1) and diethlybubblegum (2) that is 25 mole percent dimethylbubblegum (1). For each of the conditions given below find the fugacity of just dimethlybubblegum (1) in this mixture with the following data. Be sure to state and justify and assumptions used. Data for diemethlybubblegum (1): Tc=87.8CPc3=400bar=0.986Psat(at52C)=1.3barPsat(at150C)=1.8barpmat3(449C)=25barL(at52C)=0.06mol/cm3V(at1000C)=0.00134mol3V(at449C)=0.000041mol/cm3 a. (5 points) A vapor phase mixture at 150C and 2 bar. b. (10 points) A vapor phase mixture at 449C and 200 bar. c. (10 points) A liquid phase mixture at 52C and 150 bar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


