Question: please help thanks 3. Explain which ion is more stable. Why? (Hint: Use resonance.) Circle your answer. 4. Rank in order of increasing acidity 5.
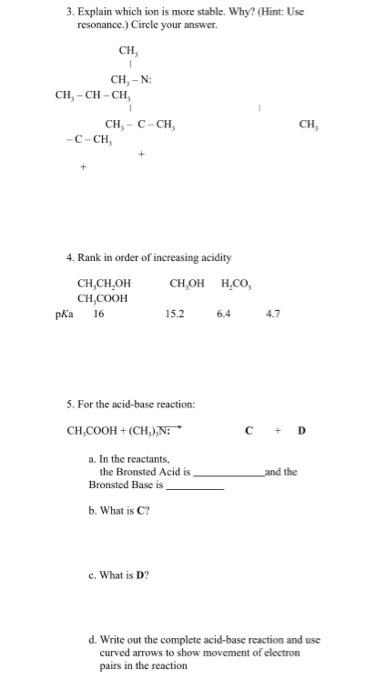
3. Explain which ion is more stable. Why? (Hint: Use resonance.) Circle your answer. 4. Rank in order of increasing acidity 5. For the acid-base reaction: CH2COOH+(CH3)3N: C+D a. In the reactants, the Bronsted Acid is and the Bronsted Base is b. What is C ? c. What is D ? d. Write out the complete acid-base reaction and use curved arrows to show movement of clectron pairs in the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


