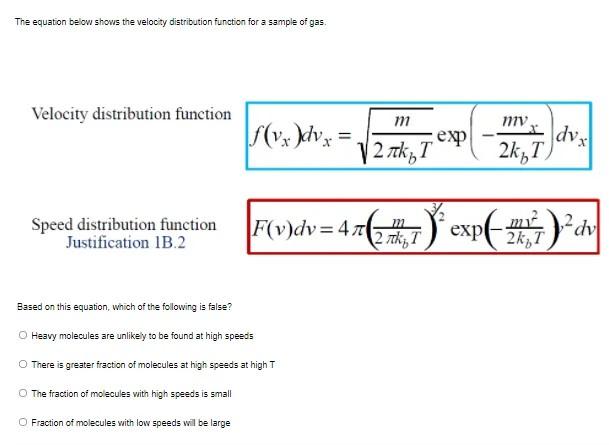
Question: please help The equation below shows the velocity distribution function for a sample of gas Velocity distribution function r f(vx)dvx m exp 12 h,T I

please help
The equation below shows the velocity distribution function for a sample of gas Velocity distribution function r f(vx)dvx m exp 12 h,T I dva 2kT) Speed distribution function Justification 1B.2 | Fv)dv=476 ) expense Didv ( m2 2 tk T Based on this equation, which of the following is false? Heavy molecules are unlikey to be found at high speeds O There is greater fraction of molecules at high speeds at high The fraction of molecules with high speeds is small Fraction of molecules with low speeds will be large Which of the following is true of the function 2 = 2(x,y) if it is a state function? The value of z depends on the amount of material present in a substance The differential of z is exact The mixed derivatives of z are equal B and C C O A, B and C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


