Question: please help The rate constant k for a certain reaction is measured at two different temperatures: Assuming the rate constant obeys the Arrhenius equation, caiculate
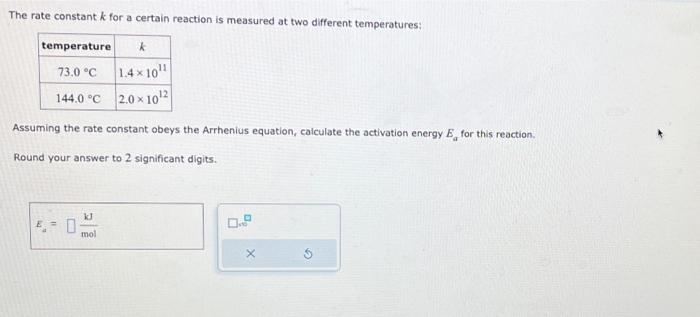
The rate constant k for a certain reaction is measured at two different temperatures: Assuming the rate constant obeys the Arrhenius equation, caiculate the activation energy Ea for this reaction. Round your answer to 2 significant digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


