Question: Please help this experiment is about transference number given the following data. Please show calculation Calculate the initial number of moles of H + (n
Please help this experiment is about transference number given the following data. Please show calculation
- Calculate the initial number of moles of H+ (ni) from 5 ml stock solution.
- Calculate the initial number of moles of H+ (ni) for total volume of the anode compartment.
- Calculate the final number of moles of H+ (nf) for 5 ml volume of anode compartment
- Calculate the final number of moles of H+ (nf) for total volume of the anode compartment.
- Calculate moles of electron (ne) theoretically
- Calculate moles of electron experimentally
- Calculate nmig theoretically
- Calculate nmig experimentally
- Calculate transference number of H+ (t+) and NO3- (t-) experimentally and theoretically.
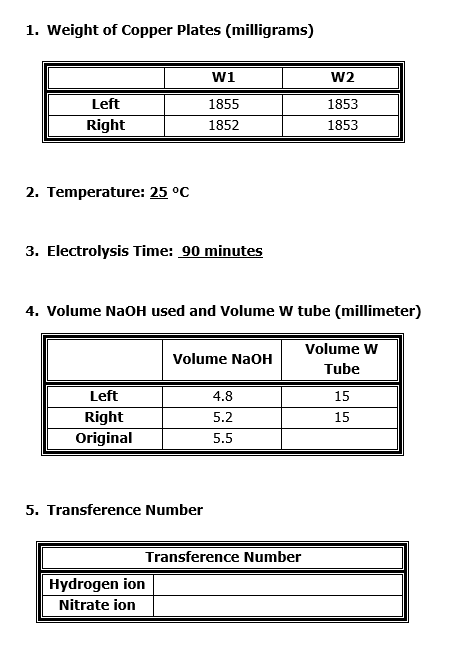
1. Weight of Copper Plates (milligrams) wi W2 Left Right 1855 1852 1853 1853 2. Temperature: 25 C 3. Electrolysis Time: 90 minutes 4. Volume NaOH used and Volume W tube (millimeter) Volume NaOH Volume w Tube Left Right Original 4.8 5.2 5.5 15 15 5. Transference Number Transference Number Hydrogen ion Nitrate ion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


