Question: please help w workings Ethyl bromide vapour decomposes by the firstorder reaction C2H5BrC2H4+HBr The activation energy is 224kJmol1 and the frequency factor is 2.31013s1. Find
please help w workings 
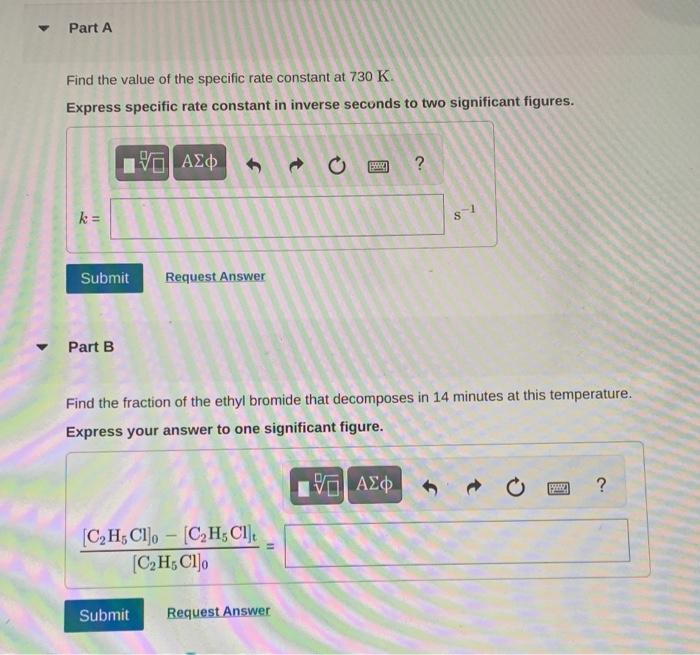
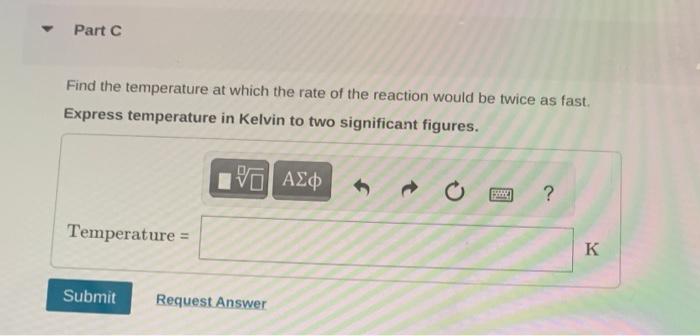



Ethyl bromide vapour decomposes by the firstorder reaction C2H5BrC2H4+HBr The activation energy is 224kJmol1 and the frequency factor is 2.31013s1. Find the value of the specific rate constant at 730K. Express specific rate constant in inverse secunds to two significant figures. Part B Find the fraction of the ethyl bromide that decomposes in 14 minutes at this temperature. Express your answer to one significant figure. Find the temperature at which the rate of the reaction would be twice as fast. Express temperature in Kelvin to two significant figures
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


