Question: Plot a full-page scatter graph with In (1/t) (on the vertical axis) vs. In [H*| (on the horizontal axis), and use a straight line
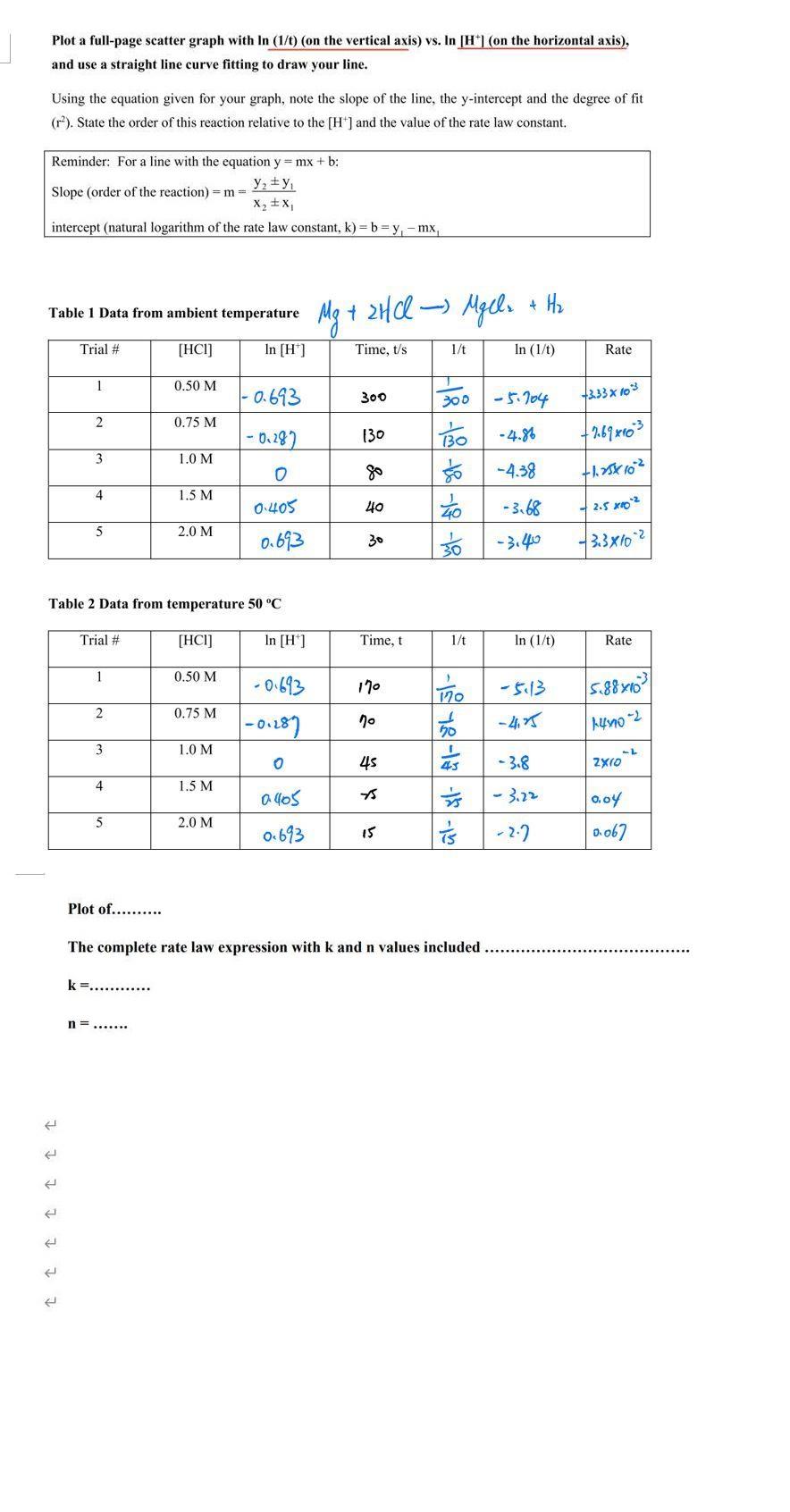
Plot a full-page scatter graph with In (1/t) (on the vertical axis) vs. In [H*| (on the horizontal axis), and use a straight line curve fitting to draw your line. Using the equation given for your graph, note the slope of the line, the y-intercept and the degree of fit (r). State the order of this reaction relative to the [H*] and the value of the rate law constant. Reminder: For a line with the equation y = mx + b: y, +y, Slope (order of the reaction) = m 3= X2 +x, intercept (natural logarithm of the rate law constant, k) = b = y, - mx, + Hz Mg + 2Hl- Table 1 Data from ambient temperature Trial # [HCI] In [H'] Time, t/s 1/t In (1/t) Rate 1 0.50 M - 0.693 -5- 104 333x 1o3 300 300 2 0.75 M -0.287 (30 BO -4.8% 3 1.0 M -4.38 4. 1.5 M to - 3.68 O-405 40 2.5 xo2 2.0 M 0.693 - 3.40 3.3x102 30 Table 2 Data from temperature 50 C Trial # [HCI] In [H'] Time, t 1/t In (1/t) Rate 1 0.50 M - 0-613 170 -5:13 170 2 0.75 M - 4, 5 3 1.0 M 45 - 3.8 4 1.5 M - 3.12 0.04 5 2.0 M o.693 - 2.7 Do67 15 Plot of... . The complete rate law expression with k and n values included k =... .
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it
To plot a scatter graph and determine the order of reaction and the rate constant follow these steps ... View full answer

Get step-by-step solutions from verified subject matter experts


