Question: please help wigh this homework question. 3. Consider your data from Table 3 (the dilution mixtures). These mixtures simulate a situation where one solution is

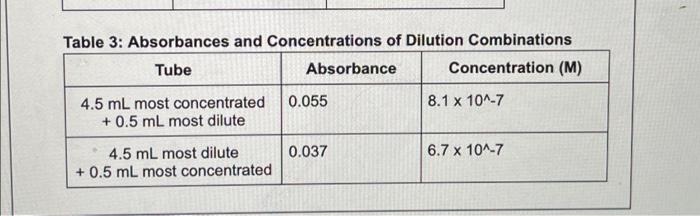
3. Consider your data from Table 3 (the dilution mixtures). These mixtures simulate a situation where one solution is contaminated by a small amount of a different solution (e.g., if you didn't change your pipette or rinse your tube). By what percent does the addition of a small quantity of dilute solution to a large quantity of concentrated solution change the molarity of the concentrated solution? What about the inverse - where a small amount of concentrated solution was added to a large amount of dilute solution? Use values determined from your work where appropriate. (1 point) Table 3: Absorbances and Concentrations of Dilution Combinations Tube Absorbance Concentration (M) 0.055 8.1 x 10^-7 4.5 mL most concentrated + 0.5 mL most dilute 0.037 6.7 x 10^-7 4.5 mL most dilute +0.5 mL most concentrated 3. Consider your data from Table 3 (the dilution mixtures). These mixtures simulate a situation where one solution is contaminated by a small amount of a different solution (e.g., if you didn't change your pipette or rinse your tube). By what percent does the addition of a small quantity of dilute solution to a large quantity of concentrated solution change the molarity of the concentrated solution? What about the inverse - where a small amount of concentrated solution was added to a large amount of dilute solution? Use values determined from your work where appropriate. (1 point) Table 3: Absorbances and Concentrations of Dilution Combinations Tube Absorbance Concentration (M) 0.055 8.1 x 10^-7 4.5 mL most concentrated + 0.5 mL most dilute 0.037 6.7 x 10^-7 4.5 mL most dilute +0.5 mL most concentrated
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


