Question: please help with 69 69. A student heated a hydrate with a formula of NaH.PO, XH,0. During the heating process water leaves the hydrate to
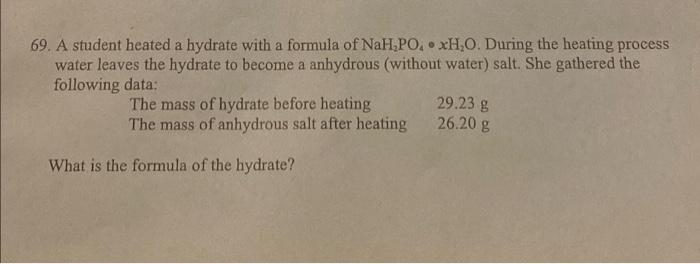
69. A student heated a hydrate with a formula of NaH.PO, XH,0. During the heating process water leaves the hydrate to become a anhydrous (without water) salt. She gathered the following data: The mass of hydrate before heating 29.23 g The mass of anhydrous salt after heating 26.20 g What is the formula of the hydrate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


