Question: please help with a lab report on density introduction, data sheet thank you Procedure Note: You can perform sections I, II, and III in any


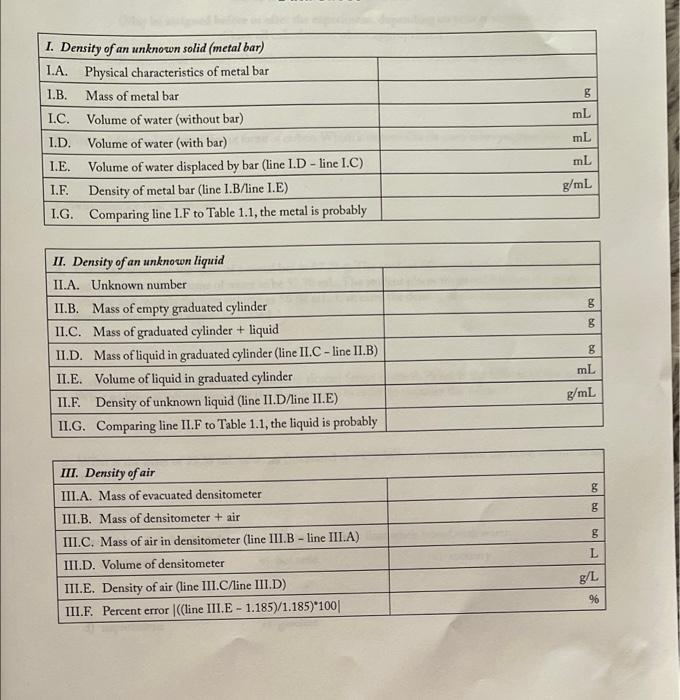
Procedure Note: You can perform sections I, II, and III in any order. I. Density of an unknown solid (metal bar) 1. Obtain a metal bar and record its color on the data sheet in pen, not pencil (data sheet line 1.A). STOP! Why is it necessary to record ALL data in pen, not pencil, and to record it on the data sheet, not on a piece of scrap paper? 2 2. Weigh the metal bar and record its exact mass on the data sheet (line 1.B). STOP! Why is it necessary to record ALL of the digits shown on the balance? 3. Place about 50 mL of water into a 100 ml graduated cylinder, read the exact volume, and record it on the data sheet (line 1.C). 4. Hold the graduated cylinder at a 45 angle and slowly slide (DO NOT DROP) the metal bar into it. STOP! What is going to happen if you drop the bar into the graduated cylinder instead of sliding it? Experiment1 Physical Properties of Matter. Density 5. Record the new volume of water in the graduated cylinder and place it on the data sheet (line 1.D). STOP! Will the number you record be greater than or less than the value you recorded in step 3? 6. Calculate the volume of water displaced by the metal bar (line L.E). 7. Calculate the density of the metal bar and record it on the data sheet (line 1.F). 8. Identify the unknown metal by comparing the calculated density (line 1.F) to the values that appear in Table 1.1, and enter its identity on line 1.G. II. Density of an unknown liquid 1. Obtain an unknown liquid and record its number on the data sheet (data sheet line ILA). 2. Weigh an empty 10 ml graduated cylinder and record its exact mass on the data sheet (line II.B). 3. Use a funnel to carefully place approximately 10 mL of the unknown liquid into the graduated cylinder and weigh the graduated cylinder with the liquid. (Between 8 and 10 mL is fine, but not more than 10 mL, as there are no markings above the 10 mL line.) Record the exact mass on the data sheet (line II.C). 4. Calculate the mass of the liquid in the graduated cylinder (line II.D). 5. Carefully read the exact volume of the liquid in the graduated cylinder and record it on the data sheet (line ILE). STOP! Why did you measure out approximately 10 mL in step3 and then record the exact volume here? 6. Calculate the density of the liquid and record it on the data sheet (line ILF). 7. Identify the unknown liquid by comparing your calculated density (line ILF) to the values that appear in Table 1.1, and enter the identity on line ILG. III. Density of air Note: Although air is a mixture of gases, the same procedure would be used for any other gas, whether pure or impure. 1. Obtain a gas densitometer from your instructor 2. Open the valve on the densitometer and attach the hose from the electric vacuum pump to the valve opening 3. Start evacuating the air by activating the pump (your instructor will demonstrate this process for you). After 1 to 2 minutes, when the pressure gauge has stopped moving, close the valve, turn off the pump, and disconnect the hose. 4. Weigh the evacuated densitometer and record the coast mass on the data sheet (data sheet linc IILA). 5. Open the valve of the densitometer and allow air to fill the vacuum (you will hear a hissing sound as air enters the evacuated vessel). 6. Weigh the densitometer again and record the exact mass on the data sheet (line III.B). STOP! Will the number you record be greater than or less than the value you recorded in step 42 7. Calculate the mass of the air in the densitometer (line III.C). 8. Enter the volume of the densitometer on the data sheet (line III.D). 9. Calculate the density of air and record it on the data sheet (line III.E). 10. Compare your calculated value to the value in Table 1.1, and calculate the percent error (line III.F). Before you leave the laboratory, be sure you can explain why your value is different than the value reported in Table 1.1. 5 1. Density of an unknown solid (metal bar) 1.A. Physical characteristics of metal bar 1.B Mass of metal bar L.C. Volume of water (without bar) I.D. Volume of water (with bar) I.E. Volume of water displaced by bar (line 1.D - line 1.C) I.F. Density of metal bar (line 1.B/line I.E) I.G. Comparing line 1.F to Table 1.1, the metal is probably mL mL mL g/mL bo 8 II. Density of an unknown liquid II.A. Unknown number II.B. Mass of empty graduated cylinder II.C. Mass of graduated cylinder + liquid II.D. Mass of liquid in graduated cylinder (line II.C - line II.B) II.E. Volume of liquid in graduated cylinder II.E Density of unknown liquid (line II.D/line II.E) II.G. Comparing line II.F to Table 1.1, the liquid is probably 8 mL g/mL S g 8 III. Density of air IIL.A. Mass of evacuated densitometer III.B. Mass of densitometer + air III.C. Mass of air in densitometer (line 111.B-line III.A) III.D. Volume of densitometer III.E. Density of air (line III.C line III.D) III.F. Percent error (line III.E - 1.185)/1.185)*100 L g/L 96
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


