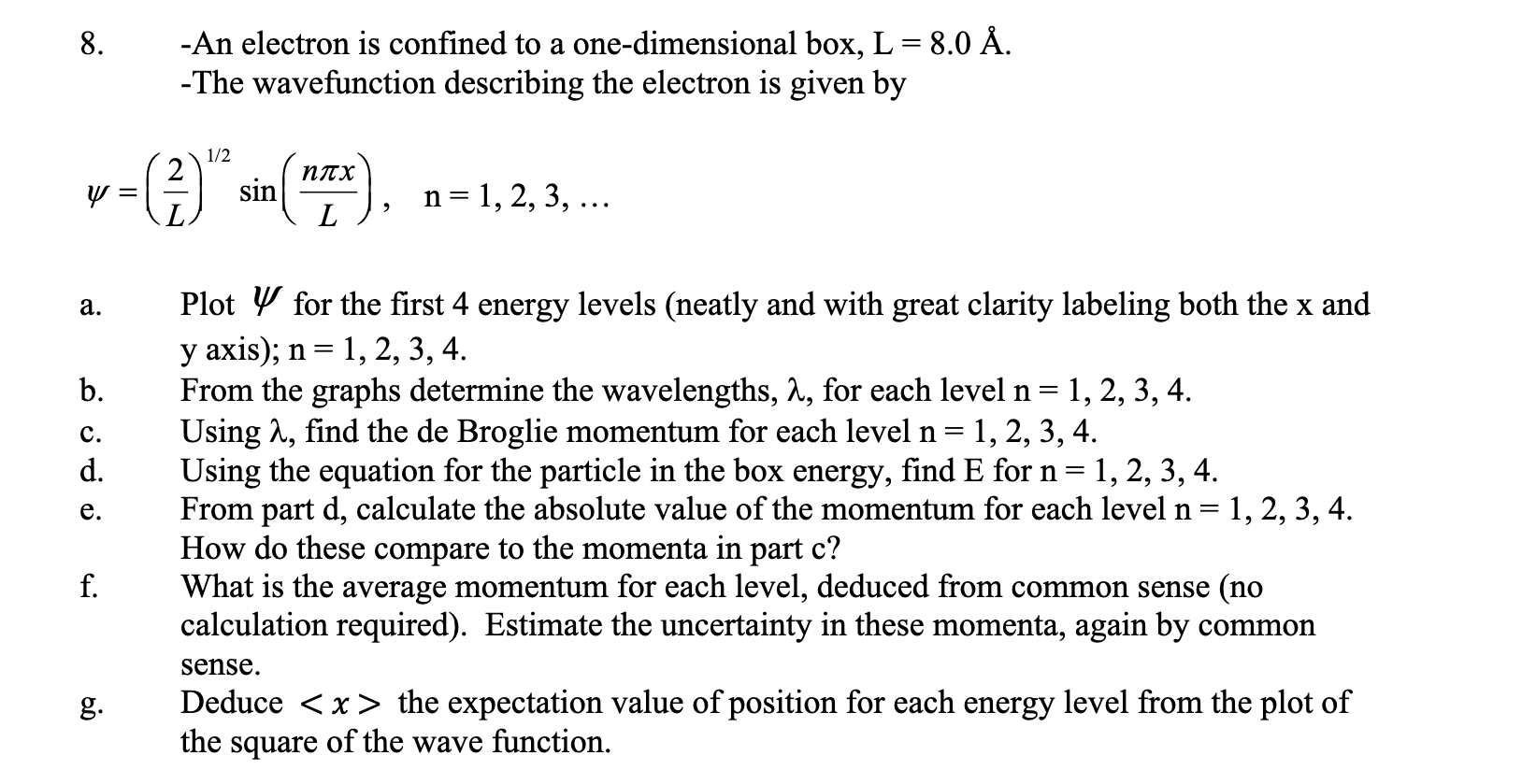
Question: Please help with a-g in as much detail as possible 8. -An electron is confined to a one-dimensional box, L=8.0A. -The wavefunction describing the electron
Please help with a-g in as much detail as possible
8. -An electron is confined to a one-dimensional box, L=8.0A. -The wavefunction describing the electron is given by =(L2)1/2sin(Lnx),n=1,2,3, a. Plot for the first 4 energy levels (neatly and with great clarity labeling both the x and y axis); n=1,2,3,4. b. From the graphs determine the wavelengths, , for each level n=1,2,3,4. c. Using , find the de Broglie momentum for each level n=1,2,3,4. d. Using the equation for the particle in the box energy, find E for n=1,2,3,4. e. From part d, calculate the absolute value of the momentum for each level n=1,2,3,4. How do these compare to the momenta in part c? f. What is the average momentum for each level, deduced from common sense (no calculation required). Estimate the uncertainty in these momenta, again by common sense. g. Deduce x the expectation value of position for each energy level from the plot of the square of the wave function
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


