Question: please help with analysis !! 1.5c Part III. Measuring Temperature Data (D) Report temperature values in C to the nearest degree. Do not enter the
 please help with analysis !!
please help with analysis !!
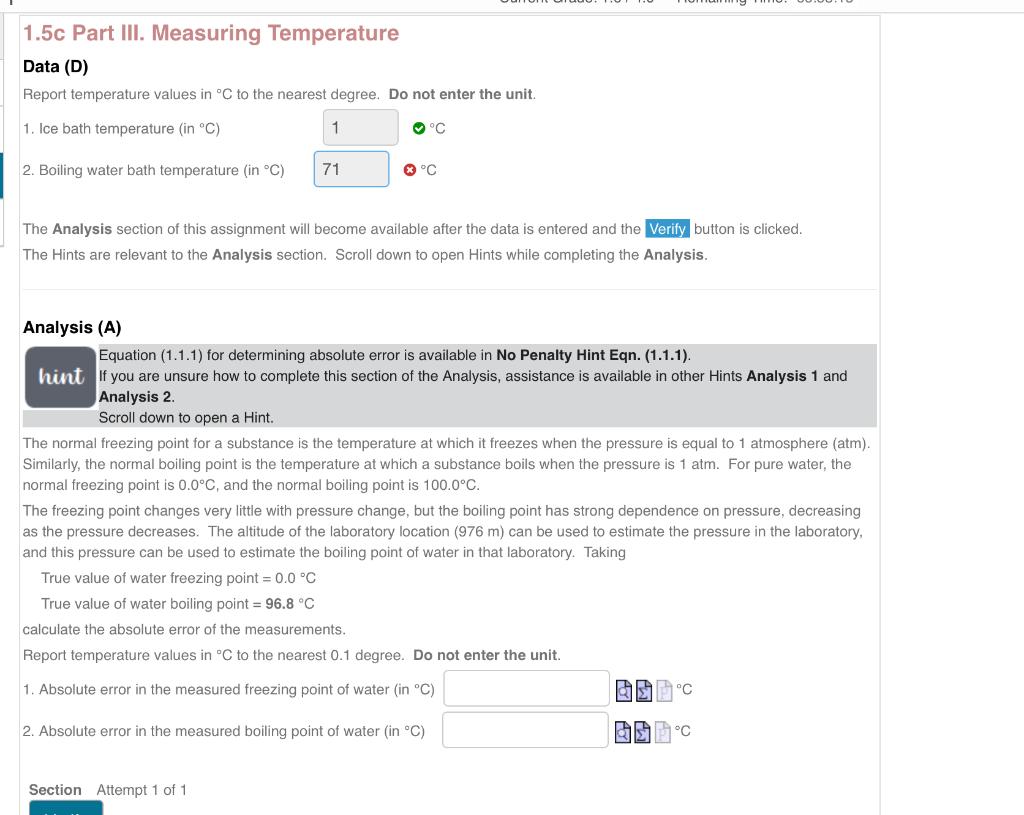
1.5c Part III. Measuring Temperature Data (D) Report temperature values in C to the nearest degree. Do not enter the unit. 1. Ice bath temperature (in C) C 2. Boiling water bath temperature ( in C) C The Analysis section of this assignment will become available after the data is entered and the button is clicked. The Hints are relevant to the Analysis section. Scroll down to open Hints while completing the Analysis. Analysis (A) Equation (1.1.1) for determining absolute error is available in No Penalty Hint Eqn. (1.1.1). If you are unsure how to complete this section of the Analysis, assistance is available in other Hints Analysis 1 and Analysis 2. Scroll down to open a Hint. The normal freezing point for a substance is the temperature at which it freezes when the pressure is equal to 1 atmosphere (atm). Similarly, the normal boiling point is the temperature at which a substance boils when the pressure is 1atm. For pure water, the normal freezing point is 0.0C, and the normal boiling point is 100.0C. The freezing point changes very little with pressure change, but the boiling point has strong dependence on pressure, decreasing as the pressure decreases. The altitude of the laboratory location (976m) can be used to estimate the pressure in the laboratory, and this pressure can be used to estimate the boiling point of water in that laboratory. Taking True value of water freezing point =0.0C True value of water boiling point =96.8C calculate the absolute error of the measurements. Report temperature values in C to the nearest 0.1 degree. Do not enter the unit. 1. Absolute error in the measured freezing point of water ( in C) C 2. Absolute error in the measured boiling point of water (inC) Q C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


