Question: please help with assignment below by showing steps of how to do questions 1-8. i do not know where to start. Trial 1 [A] mol-1

![[A] mol-1 0.431 0.690 1.931 2 3 B) mol11 0.427 0.427 0.769](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f84db8c78a8_85666f84db86ad35.jpg)
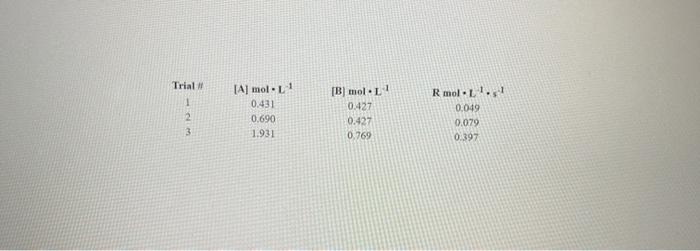
Trial 1 [A] mol-1 0.431 0.690 1.931 2 3 B) mol11 0.427 0.427 0.769 Rmol. 0.049 0.079 0.397 In this experiment, you will mix simulated reactants "A" and "8* and measure the rate of formation of "C" according to the equation: A+Bc Using the data provided, you will calculate the orders with respect to "A" and "8", as well as the rate constant Now, go to the online lab to see the results: ONLINE LAB Questions: 1. What is the order of your reaction with respect to A? Show your work in the space below. 5 2. What is the order of your reaction with respect to B? Show your work in the space below. 3. Type the differential rate law in the space below. Use the format R = K[Al"(B)". Doint 4. What is the rate constant, including units, of your rate law? Show your work in the space below to 5. Below are nine potential reaction mechanisms for your reaction Select the one that is consistent with your laboratory data. Indicate your selection by typing the number here Option 1 Option 2 Option 3 A+ A-D slow DBC-A fast slow BBD DEA-C A.BD slow Option 4 Option 5 Option 2A+B-D D-CA slow fast 2B - AD D-C+B slow 2A + 2B --D D.C.A.B slow fast Option 7 Option Option 9 9 R slow AD DBC BD DEAC fast slow fast 2A-38-D slow DC.A. 28 fast 6 F 6. Describe how you determined your answer for question. Please be thorough. 3 7. What do you expect would be the effect on your rate constant if this experiment were performed 50C rather than at room temperature (25C). Why would you expect this change? Please be extremely specific in your answer. 8. Suppose an alternate reaction were developed to produce "C" F+G-C and that this reaction was second order with respect to "Fand third order with respect to "G". Do you think this reaction would be a faster way to make "C" than the reaction in your lab? Is there any information you would need in order to better answer this question? This question is trickier than you might think. Take your time and be extremely specific in your answer.noin
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


