Question: please help with both questions! Exercise 3 Bonus. Using your calculations from Exercise 2, complete the overlap matrix. (28|2s) (2px/2s) (2py|2s) S= (2px|2s) (2px|2px) (2px|2py)

 please help with both questions!
please help with both questions!
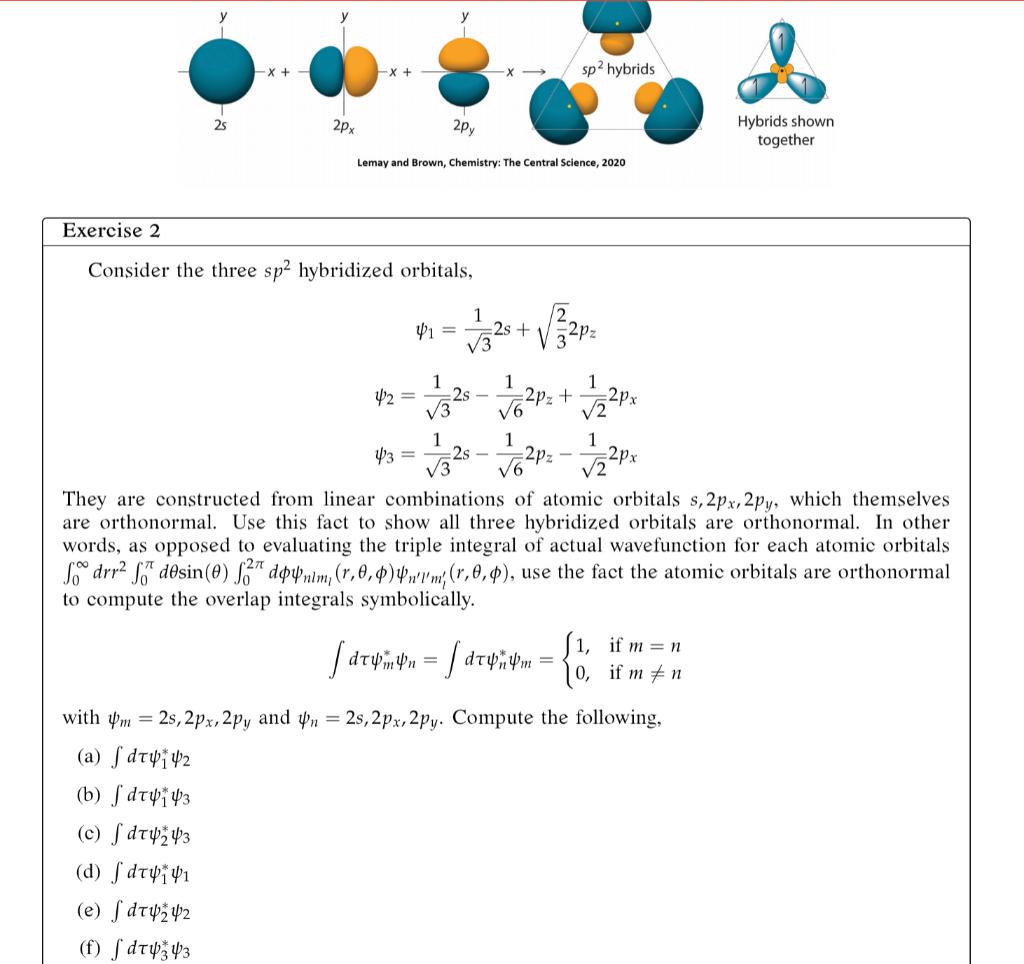
Exercise 3 Bonus. Using your calculations from Exercise 2, complete the overlap matrix. (28|2s) (2px/2s) (2py|2s) S= (2px|2s) (2px|2px) (2px|2py) (2py|2s) (2py|2px) (2py|2py)) The rules for Bra-Ket notation are in Lab 5 and just a symbolic way of abbreviating your calculations in Exercise 2. Note. (m ) = (n|m)* X + -X + sp2 hybrids 25 2px 2py Hybrids shown together Lemay and Brown, Chemistry: The Central Science, 2020 Exercise 2 Consider the three sp2 hybridized orbitals, 1 41 = V3 -25 + 20: 1 =2px 1 1 42 = 2s 2pz + V3 V6 V2 1 1 1 43 = V3 2s 5328 - 5o2pz - 122px V2 They are constructed from linear combinations of atomic orbitals s, 2px, 2py, which themselves are orthonormal. Use this fact to show all three hybridized orbitals are orthonormal. In other words, as opposed to evaluating the triple integral of actual wavefunction for each atomic orbitals Sdrr2 S7" dsin(0) 13" donm, (7,0,0); (r,0,0), use the fact the atomic orbitals are orthonormal to compute the overlap integrals symbolically. i, if m=n d facturintin = drydym = {0, if m #n with 4m = 25, 2px, 2py and yn = 2s, 2px,2py. Compute the following, (a) dt4142 (b) S dt4i43 (c) S dTUY3 (d) dtvi 41 (e) dT42 42 (f) dT4343
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


