Question: Please help with detail on how to solve these! Chris Consider a compound with the molecular formula C6H10O a) How many degrees of unsaturation does
Please help with detail on how to solve these!
Chris 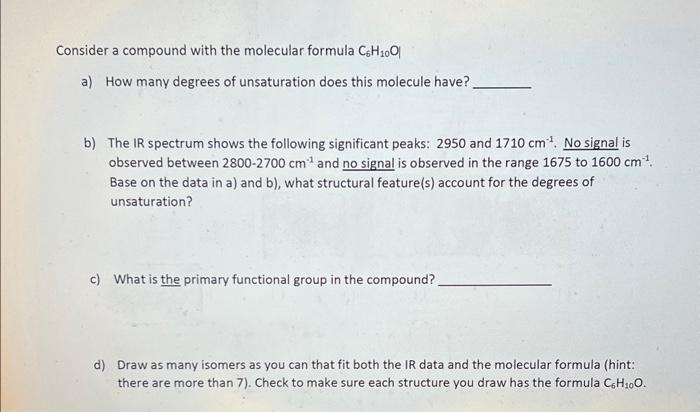

Consider a compound with the molecular formula C6H10O a) How many degrees of unsaturation does this molecule have? b) The IR spectrum shows the following significant peaks: 2950 and 1710cm1. No signal is observed between 28002700cm1 and no signal is observed in the range 1675 to 1600cm1. Base on the data in a) and b), what structural feature(s) account for the degrees of unsaturation? c) What is the primary functional group in the compound? d) Draw as many isomers as you can that fit both the IR data and the molecular formula (hint: there are more than 7). Check to make sure each structure you draw has the formula C6H10O
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


