Question: Please help with e and f Problem 2 (20 points). Consider the following thermodynamic cycle: E+S+1 #ES +1 11 ht EI+SFESI Clearly, the cycle is



Please help with e and f
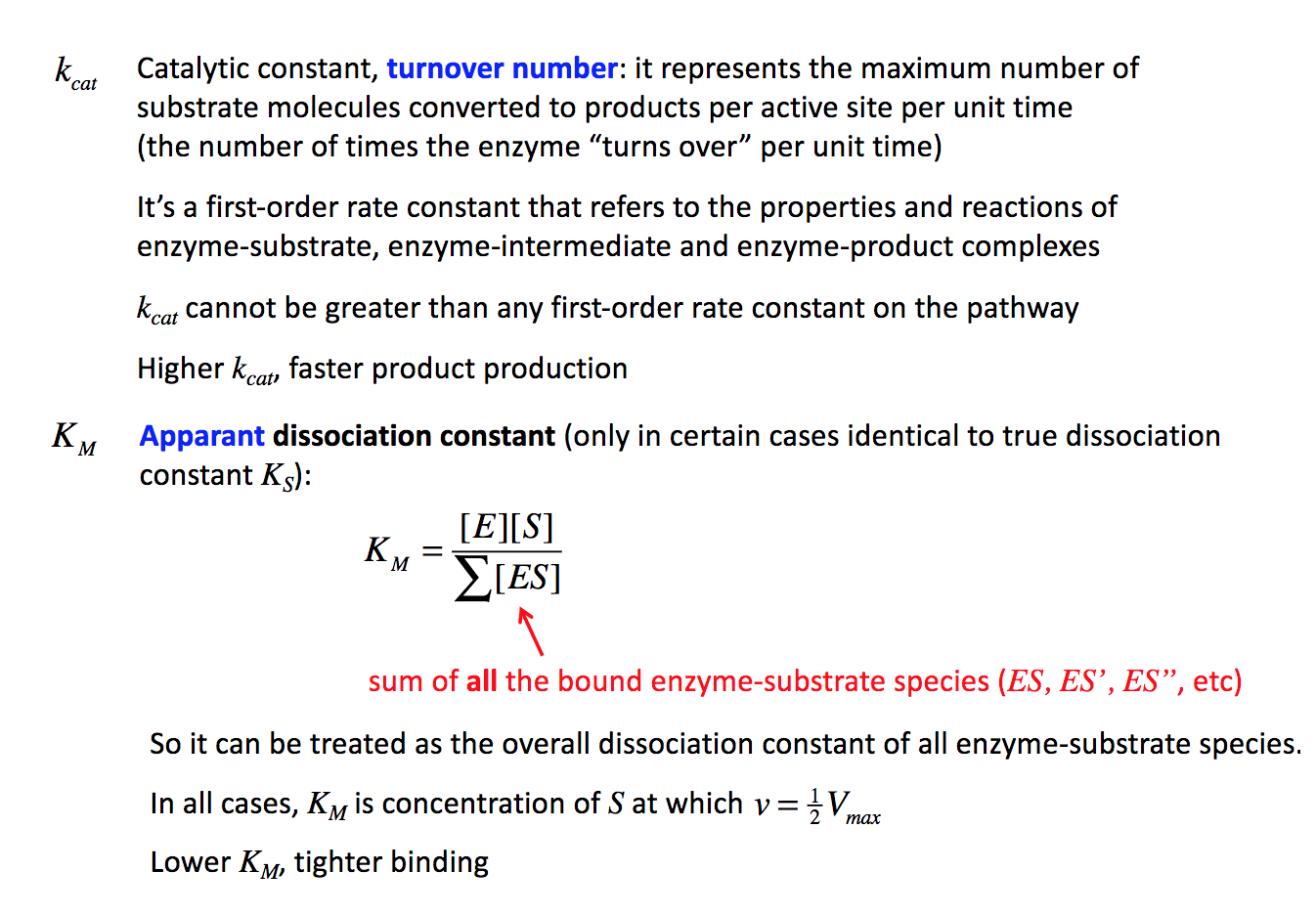
Problem 2 (20 points). Consider the following thermodynamic cycle: E+S+1 #ES +1 11 ht EI+SFESI Clearly, the cycle is specified by four different dissociation constants (1 per reaction). In this cycle, let's indicate the following directions by letters a-d: E+S+IES+I ^ ATT 1. b EI +S ESI = Using this notation, AG, = G(ES)+G(I)-G(E)-G(S)-G(I), while AG, = G(E)+G(S)+G(I)-G(EI) G(S), etc. (thus, head of arrow minus tail of arrow). = = e (5 points). Generalizing the interpretation of Ky on slide 17 of enzyme_kinetics.pdf to a situation where inhibitors are present: [S] (free E) KM (ES) where the sum on top indicates a sum of all non-substrate bound enzyme species (e.g. E but also EI), and the sum in the bottom a sum of all substrate-bound enzyme species. Starting from this equation, derivate mathematically that noncompetitive binding does not affect Ky f(7 points). Now use the Michaelis-Menten scheme to derive kca and K, for noncompetitive binding; start from scratch and do not use your result from 2e. M. kcat Catalytic constant, turnover number: it represents the maximum number of substrate molecules converted to products per active site per unit time (the number of times the enzyme turns over per unit time) It's a first-order rate constant that refers to the properties and reactions of enzyme-substrate, enzyme-intermediate and enzyme-product complexes kcat cannot be greater than any first-order rate constant on the pathway KM Higher kcat, faster product production Apparant dissociation constant (only in certain cases identical to true dissociation constant Ks): [E][S] K [ES] M sum of all the bound enzyme-substrate species (ES, ES', ES, etc) So it can be treated as the overall dissociation constant of all enzyme-substrate species. In all cases, Ky is concentration of S at which v= { Vmax Lower Km, tighter binding = M Problem 2 (20 points). Consider the following thermodynamic cycle: E+S+1 #ES +1 11 ht EI+SFESI Clearly, the cycle is specified by four different dissociation constants (1 per reaction). In this cycle, let's indicate the following directions by letters a-d: E+S+IES+I ^ ATT 1. b EI +S ESI = Using this notation, AG, = G(ES)+G(I)-G(E)-G(S)-G(I), while AG, = G(E)+G(S)+G(I)-G(EI) G(S), etc. (thus, head of arrow minus tail of arrow). = = e (5 points). Generalizing the interpretation of Ky on slide 17 of enzyme_kinetics.pdf to a situation where inhibitors are present: [S] (free E) KM (ES) where the sum on top indicates a sum of all non-substrate bound enzyme species (e.g. E but also EI), and the sum in the bottom a sum of all substrate-bound enzyme species. Starting from this equation, derivate mathematically that noncompetitive binding does not affect Ky f(7 points). Now use the Michaelis-Menten scheme to derive kca and K, for noncompetitive binding; start from scratch and do not use your result from 2e. M. kcat Catalytic constant, turnover number: it represents the maximum number of substrate molecules converted to products per active site per unit time (the number of times the enzyme turns over per unit time) It's a first-order rate constant that refers to the properties and reactions of enzyme-substrate, enzyme-intermediate and enzyme-product complexes kcat cannot be greater than any first-order rate constant on the pathway KM Higher kcat, faster product production Apparant dissociation constant (only in certain cases identical to true dissociation constant Ks): [E][S] K [ES] M sum of all the bound enzyme-substrate species (ES, ES', ES, etc) So it can be treated as the overall dissociation constant of all enzyme-substrate species. In all cases, Ky is concentration of S at which v= { Vmax Lower Km, tighter binding = M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


