Question: Please help with my questions. Overview/Introduction In this assignment, you will determine the conjugate acid or base in acid-base reactions. This will help you understand
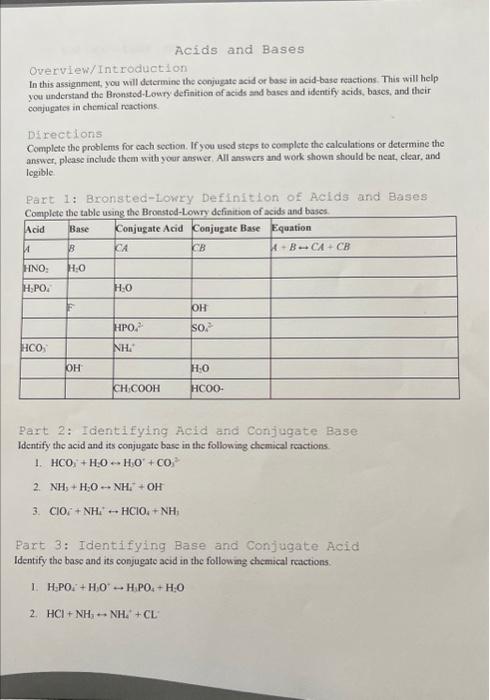

Overview/Introduction In this assignment, you will determine the conjugate acid or base in acid-base reactions. This will help you understand the Bronsted-Lowry definition of acids and bases and identify acids, bascs, and their coajugates in chemical reactions. Directions Complete the problems for cach section. If you used steps to complete the calculations or determine the answer, please include them with yoor answer, All answers and work shown should be neat, clear, and legible. Part 1: Bronsted-lowry Definition of Aclds and Bases Pazt 2: Identifying Acid and Conjugate Base Identify the acid and its conjugate base in the following chemical reactions. 1. HCO1++H2OH1O++CO2 2. NH3+H2ONH4++OH 3. ClOi+NH4HClO4+NH3 Part 3: Identifying Base and Conjugate Aci.d Identify the base and its conjugate acid in the following chemical renctions. I. H3PO4++H3O+H1PO4+H=O 2. HCl+NH7NH2++CL 1. A drug is considered to be "eliminated" from the body after 5 half-lives. How much iodine-131 would remain in Mr. Miller's body after that period of time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


