Question: please help with part a, b, and c Alka-Seltzer is an over-the-counter medicine used to treat acid indigestion and heartburn. The active pharmaceutical ingredients (API)
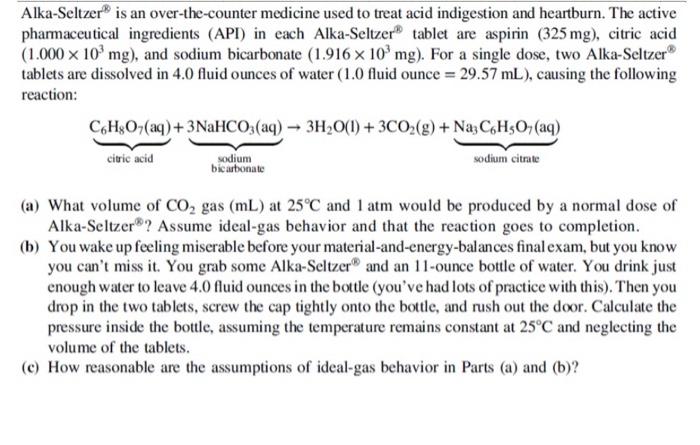
Alka-Seltzer is an over-the-counter medicine used to treat acid indigestion and heartburn. The active pharmaceutical ingredients (API) in each Alka-Seltzer 20 tablet are aspirin (325mg), citric acid (1.000103mg), and sodium bicarbonate (1.916103mg). For a single dose, two Alka-Seltzer 8 tablets are dissolved in 4.0 fluid ounces of water (1.0 fluid ounce =29.57mL), causing the following reaction: (a) What volume of CO2 gas (mL) at 25C and 1atm would be produced by a normal dose of Alka-Seltzer 8 ? Assume ideal-gas behavior and that the reaction goes to completion. (b) You wake up feeling miserable before your material-and-energy-balances final exam, but you know you can't miss it. You grab some Alka-Seltzer and an 11-ounce bottle of water. You drink just enough water to leave 4.0 fluid ounces in the bottle (you've had lots of practice with this). Then you drop in the two tablets, screw the cap tightly onto the bottle, and rush out the door. Calculate the pressure inside the bottle, assuming the temperature remains constant at 25C and neglecting the volume of the tablets. (c) How reasonable are the assumptions of ideal-gas behavior in Parts (a) and (b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


