Question: Please help with part B, ive been struggling to solve this for a while and cant seem to understand it. it would be greatly appreciated

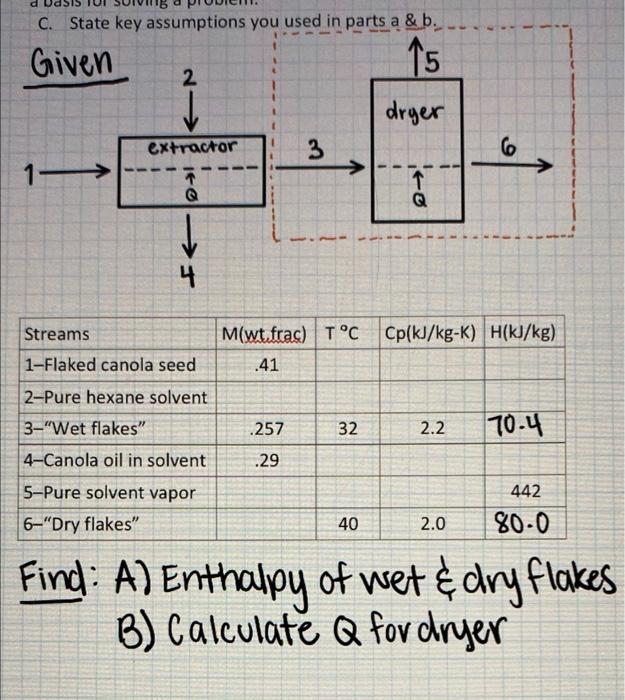
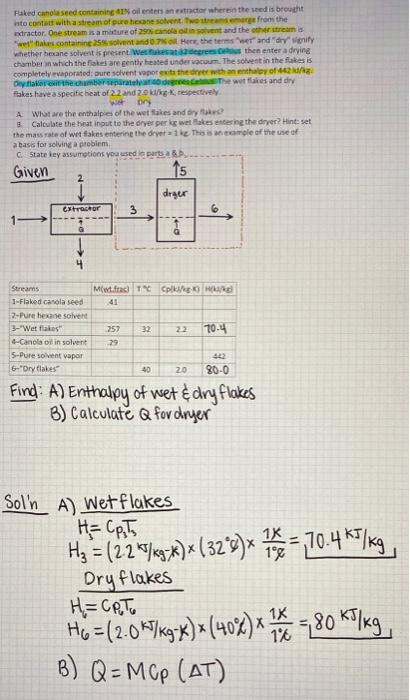
a Flaked canola seed containing 41% oil enters an extractor wherein the seed is brought into contact with a stream of pure hexane solvent. Two streams emerge from the extractor. One stream is a mixture of 29% canola oil in solvent and the other stream is "wet" flakes containing 25% solvent and 0.7% oil. Here, the terms "wet" and "dry" signify whether hexane solvent is present. Wet flakes at 32 degrees Celsius then enter a drying chamber in which the flakes are gently heated under vacuum. The solvent in the flakes is completely evaporated; pure solvent vapor exits the dryer with an enthalpy of 442 kJ/kg. Dry flakes exit the chamber separately at 40 degrees Celsius. The wet flakes and dry flakes have a specific heat of 2.2 and 2.0 kJ/kg-K, respectively. Wet Dry A. What are the enthalpies of the wet flakes and dry flakes? B. Calculate the heat input to the dryer per kg wet flakes entering the dryer? Hint: set the mass rate of wet flakes entering the dryer = 1 kg. This is an example of the use of a basis for solving a problem. C. State key assumptions you used in parts a & b. Given 2 15 15 C. State key assumptions you used in parts a & b. Given 5 2 dryer extractor 3 1 1 Q 6 10-> 4 M(wt.frac) TC Cp(kJ/kg-K) H(kJ/kg) Streams 1-Flaked canola seed .41 2-Pure hexane solvent 3-"Wet flakes" .257 32 2.2 70.4 .29 4-Canola oil in solvent 5-Pure solvent vapor 6"Dry flakes" 442 40 2.0 80.0 Find: A) Enthalpy of wet & dry flakes B) Calculate Q for dryer Raked canolaseed containing Nolters an actor where the seed is brought into contact with a team of pure honest women from the odractor. One stream is a midure of Scandinavent and the other team is "Welles containing 25 solventand. Here, the test and diniy whether hexane solvent is present. We are then enter a drying chamber which the files are gently heated under acom. The solvent in the stakes is completely evaporated: pure solvent vapor exits there with an enthalpy of diy falleciderethe wet takes and dry Flakes have a specific heat of 22 and 20k/kg-K, respectively Wo ory A What are the enthalples of the wet takes and dry Calculate the heat input to the dryer per kg wet les entering the dryer? Hint: set the mass rate of wet fakes entering the dryer 1 This emple of the use of a basis for solving problem Stateley assumptions you used parts Given drger Ctractor 3 1 ed Streams Mw.fac) TCH 1-Flaked canolaseed 41 2- Pure hexane solvent 3-"Wet flakes 257 32 22 70-4 4-Canola oil insolvent 29 5-Pure solvent vapor 6- Dry takes 40 20 20-0 Find: A) Enthalpy of wet & dry flakes B) Calculate Q for dryer 1K Sol'n A) Wet flakes = , H2 = (2.2%7ky:X)* (32*9)x 18 = 70.467/kg, Dry flakes H=CATO H6=(2.0k/kg-x) = (40%) X 7 - 12 = 80 kJ/kg (%* B) Q=MCP (AT) 1x 1% ,, a Flaked canola seed containing 41% oil enters an extractor wherein the seed is brought into contact with a stream of pure hexane solvent. Two streams emerge from the extractor. One stream is a mixture of 29% canola oil in solvent and the other stream is "wet" flakes containing 25% solvent and 0.7% oil. Here, the terms "wet" and "dry" signify whether hexane solvent is present. Wet flakes at 32 degrees Celsius then enter a drying chamber in which the flakes are gently heated under vacuum. The solvent in the flakes is completely evaporated; pure solvent vapor exits the dryer with an enthalpy of 442 kJ/kg. Dry flakes exit the chamber separately at 40 degrees Celsius. The wet flakes and dry flakes have a specific heat of 2.2 and 2.0 kJ/kg-K, respectively. Wet Dry A. What are the enthalpies of the wet flakes and dry flakes? B. Calculate the heat input to the dryer per kg wet flakes entering the dryer? Hint: set the mass rate of wet flakes entering the dryer = 1 kg. This is an example of the use of a basis for solving a problem. C. State key assumptions you used in parts a & b. Given 2 15 15 C. State key assumptions you used in parts a & b. Given 5 2 dryer extractor 3 1 1 Q 6 10-> 4 M(wt.frac) TC Cp(kJ/kg-K) H(kJ/kg) Streams 1-Flaked canola seed .41 2-Pure hexane solvent 3-"Wet flakes" .257 32 2.2 70.4 .29 4-Canola oil in solvent 5-Pure solvent vapor 6"Dry flakes" 442 40 2.0 80.0 Find: A) Enthalpy of wet & dry flakes B) Calculate Q for dryer Raked canolaseed containing Nolters an actor where the seed is brought into contact with a team of pure honest women from the odractor. One stream is a midure of Scandinavent and the other team is "Welles containing 25 solventand. Here, the test and diniy whether hexane solvent is present. We are then enter a drying chamber which the files are gently heated under acom. The solvent in the stakes is completely evaporated: pure solvent vapor exits there with an enthalpy of diy falleciderethe wet takes and dry Flakes have a specific heat of 22 and 20k/kg-K, respectively Wo ory A What are the enthalples of the wet takes and dry Calculate the heat input to the dryer per kg wet les entering the dryer? Hint: set the mass rate of wet fakes entering the dryer 1 This emple of the use of a basis for solving problem Stateley assumptions you used parts Given drger Ctractor 3 1 ed Streams Mw.fac) TCH 1-Flaked canolaseed 41 2- Pure hexane solvent 3-"Wet flakes 257 32 22 70-4 4-Canola oil insolvent 29 5-Pure solvent vapor 6- Dry takes 40 20 20-0 Find: A) Enthalpy of wet & dry flakes B) Calculate Q for dryer 1K Sol'n A) Wet flakes = , H2 = (2.2%7ky:X)* (32*9)x 18 = 70.467/kg, Dry flakes H=CATO H6=(2.0k/kg-x) = (40%) X 7 - 12 = 80 kJ/kg (%* B) Q=MCP (AT) 1x 1%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


